What Is The General Form Of A Synthesis Reaction
What Is The General Form Of A Synthesis Reaction - A + b → ab. In most cases, synthesis reactions release energy. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. The general form of a combination reaction is: Web in a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. A + b → ab. Web this is the general form of a synthesis reaction. Substitution or single replacement reactions a single free element replaces or is substituted for one of the elements in a compound. Sodium and chlorine ions interact to form sodium chloride. 4 al (s) + 3 o 2 (g) → 2 al 2 o 3 (s) iron sulfide:
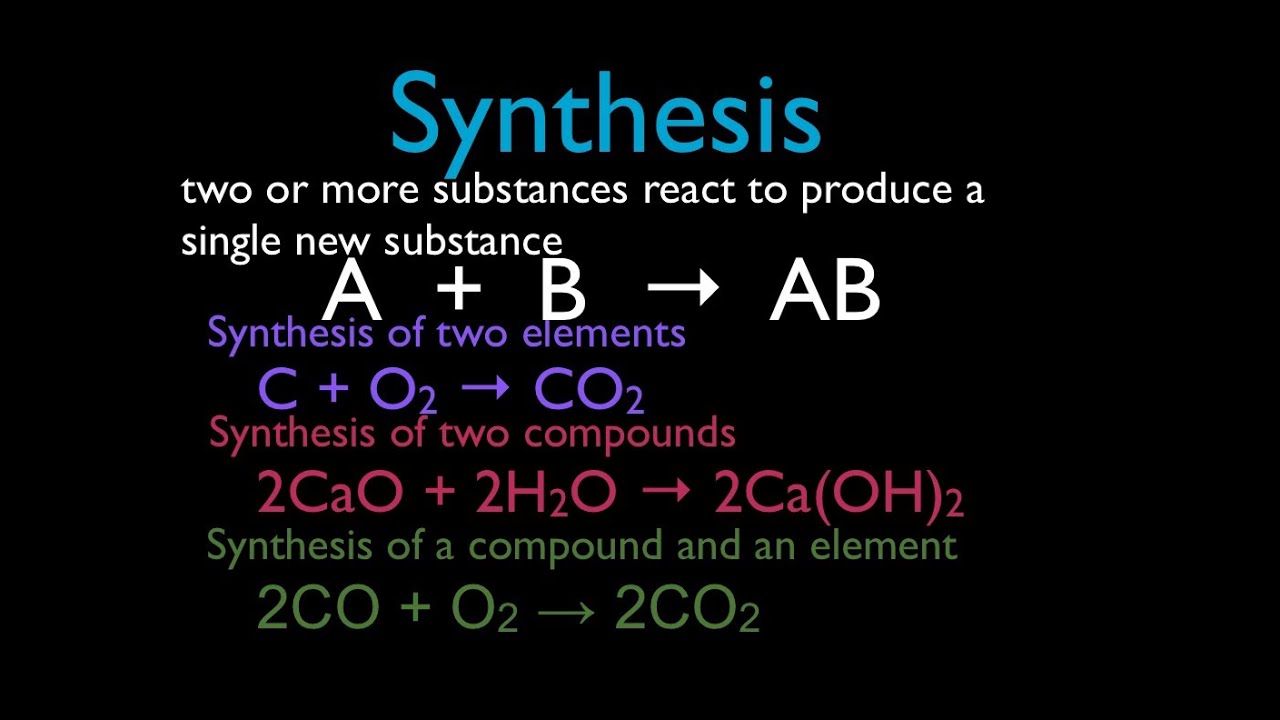
4 al (s) + 3 o 2 (g) → 2 al 2 o 3 (s) iron sulfide: Web in a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. 8 fe + s 8 → 8 fes. A + b → ab. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Web a synthesis reaction occurs when two or more reactants combine to form a single product. Reactions that release energy are considered exothermic. A synthesis reaction is the opposite of a decomposition reaction, which. Web a synthesis reaction canbe represented by the general equation: Combination or synthesis reactions two or more reactants unite to form a single product.
Web synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Synthesis reactions put things together. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. The arrow shows the direction in which the reaction occurs. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. Solid sodium metal reacts with chlorine gas to produce solid sodium chloride. Ab → a + b consider the decomposition of calcium carbonate: Caco 3 (s) → cao (s) + co 2 (g) calcium carbonate calcium oxide carbon dioxide synthesis reactions a synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. A + b → ab. A + b → ab.
Synthesis Reactions YouTube
Web a synthesis reaction, also known as a direct combination or combination reaction, is a chemical process in which two or more simple elements or compounds combine to form a more complex product. These reactions come in the general form of: 3 h 2 (g) + n 2 (g) → 2 nh 3 (g) aluminum oxide: Web a synthesis reaction.
Main Kinds of Chemical Reactions
A + b → ab Web in chemistry, chemical synthesis ( chemical combination) is the artificial execution of chemical reactions to obtain one or several products. The general form of a combination reaction is: Web the general form of a synthesis reaction is a + b → ab. It is represented by the equation:
Main Kinds of Chemical Reactions
In a synthesis reaction, two or more chemical species combine to form a more complex product. In this form, a synthesis reaction is easy to recognize because you have more reactants than products. A + b → ab. Web a synthesis reaction, also known as a direct combination or combination reaction, is a chemical process in which two or more.
Types of Chemical Reactions Synthesis reaction
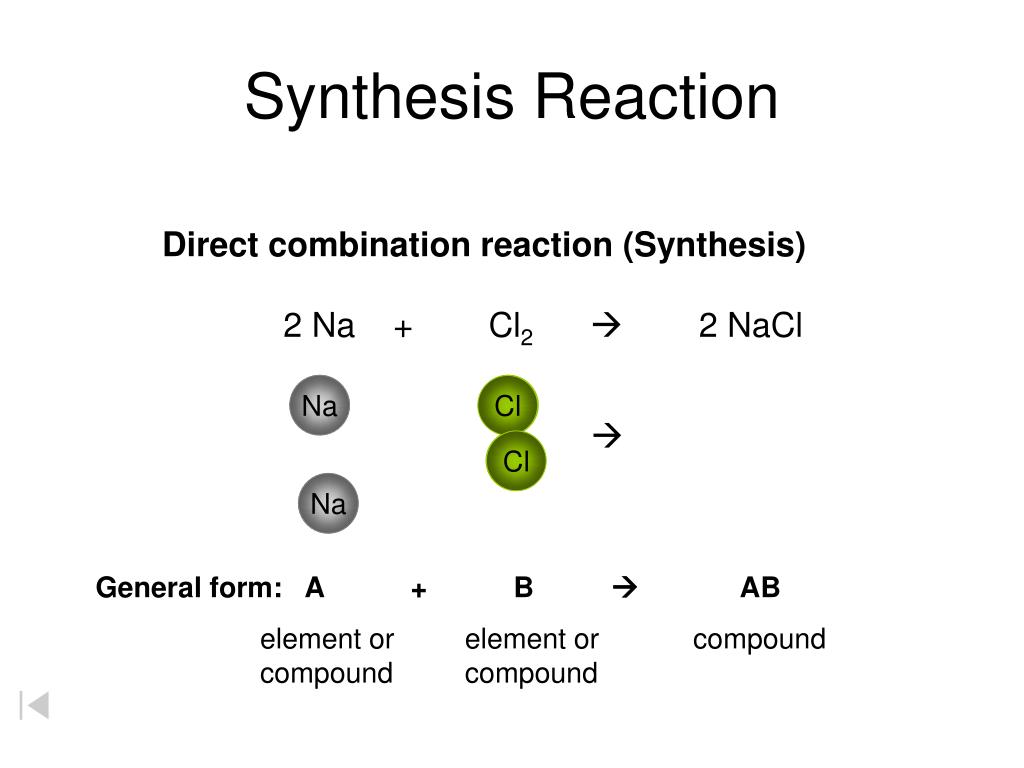
2na(s) + cl 2(g) → 2nacl(s) The basic form of a synthesis reaction is: This type of reaction is represented by the general equation: The combination of iron and sulfur to form iron (ii) sulfide is an example of a synthesis reaction: They involve the formation of either ionic or covalent bonds.
PPT Chemical Equations & Reactions PowerPoint Presentation, free
It is also called a direct reaction and is one of the most common chemical reactions. Combination or synthesis reactions two or more reactants unite to form a single product. The combination of iron and sulfur to form iron (ii) sulfide is an example of a synthesis reaction: Solid sodium metal reacts with chlorine gas to produce solid sodium chloride..
Types of chemical reactions
2na(s) + cl 2(g) → 2nacl(s) Web combination reactions can also be called synthesis reactions. In most cases, synthesis reactions release energy. 3 h 2 (g) + n 2 (g) → 2 nh 3 (g) aluminum oxide: A synthesis reaction is the opposite of a decomposition reaction, which.
Synthesis Reactions — Definition & Examples Expii
One of the main types of chemical reactions is a synthesis or direct combination reaction. A + b → ab. Web the general form of a synthesis reaction is: A+ b → ab one combination reaction is two elements combining to form a compound. Combination reactions can also be called synthesis reactions.
Dehydration Synthesis Definition, Examples, and Equations
3 h 2 (g) + n 2 (g) → 2 nh 3 (g) aluminum oxide: One of the main types of chemical reactions is a synthesis or direct combination reaction. In a synthesis reaction, two or more chemical species combine to form a more complex product. Web the general form of a synthesis reaction is a + b → ab..
What Is a Synthesis Reaction? Definition and Examples
Web a synthesis reaction occurs when two or more reactants combine to form a single product. One example of a synthesis reaction is the combination of iron (fe) and sulfur (s) to form iron sulfide. A + b → ab. It's easy to spot when one of the reactants is an element. A + b → ab.
🎉 Which is an example of synthesis. 2 Synthesis Essay Examples That
This type of reaction is represented by the general equation: Combination or synthesis reactions two or more reactants unite to form a single product. These reactions come in the general form of: Web the general form of a synthesis reaction is: Combination reactions can also be called synthesis reactions.
When The Two Or More Reactants Combine They Make A Larger Compound.
A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. Web the general equation represents this type of reaction: A+ b → ab one combination reaction is two elements combining to form a compound. Web a synthesis reaction, also known as a direct combination or combination reaction, is a chemical process in which two or more simple elements or compounds combine to form a more complex product.
As The Name Implies, Simple Reactants Make Or Synthesize A More Complex Product.
Web a synthesis reaction is when two or more simple compounds combine to form a more complicated one. Web in a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. Web a synthesis reaction is when the compounds or elements combine and turn into a new compound. 2na(s) + cl 2(g) → 2nacl(s)
[1] This Occurs By Physical And Chemical Manipulations Usually Involving One Or More Reactions.
The chemical equation for a general form of synthesis reaction is as follows: A typical example of a synthesis reaction is the formation of table salt. A + b → ab. One of the main types of chemical reactions is a synthesis or direct combination reaction.
4 Al (S) + 3 O 2 (G) → 2 Al 2 O 3 (S) Iron Sulfide:
Web combination reactions can also be called synthesis reactions.the general form of a combination reaction is: In most cases, synthesis reactions give off heat, so they are considered exothermic. The arrow shows the direction in which the reaction occurs. Web the general form for a decomposition reaction is:
:max_bytes(150000):strip_icc()/synthesis_reaction-56a1327a3df78cf7726851a5.png)
:max_bytes(150000):strip_icc()/single_displacement_reaction-56a1327a3df78cf7726851ad.png)