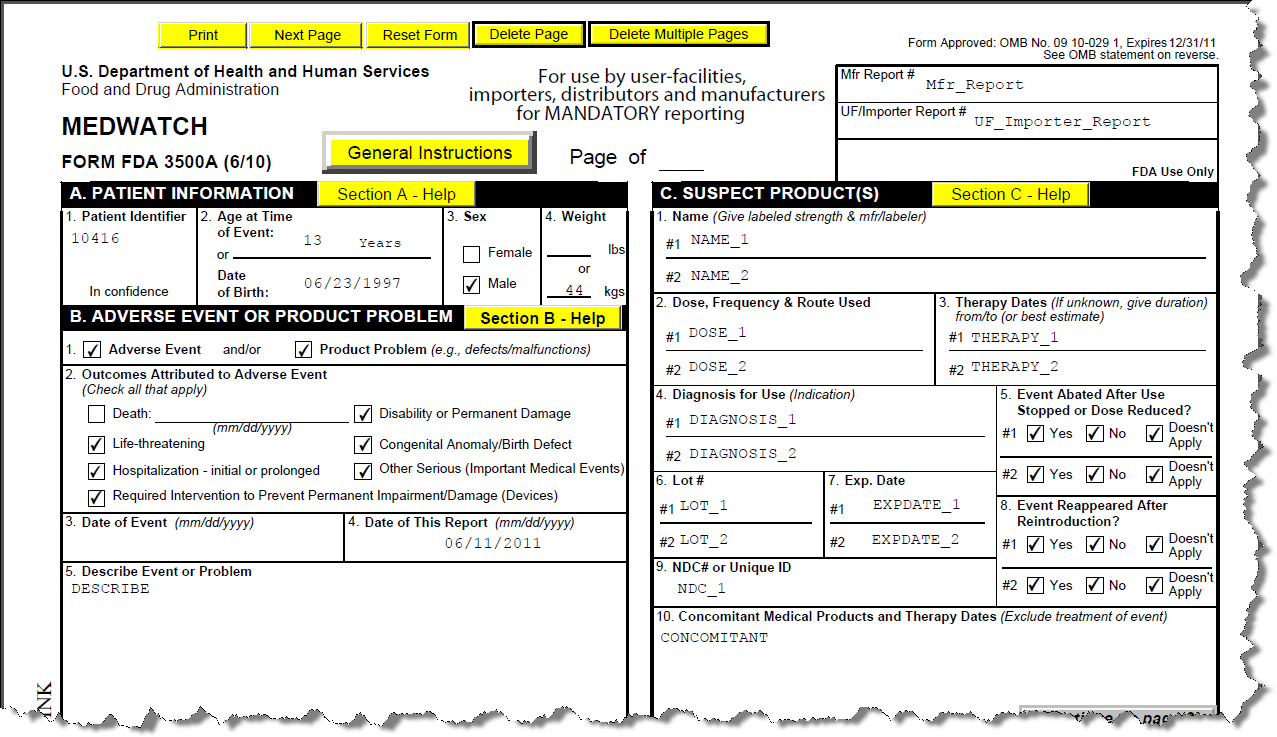
Med Watch Form
Med Watch Form - Easily fill out pdf blank, edit, and sign them. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Safety alerts for human medical products (drugs, biologics. Web complete medwatch form online with us legal forms. For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web a copy of a dhmh medwatch form and instructions are available at the links below. Save or instantly send your ready documents. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Patient or patient healthcare representative: Web online using the medwatch online reporting form;
Web a copy of a dhmh medwatch form and instructions are available at the links below. Patient or patient healthcare representative: Save or instantly send your ready documents. Easily fill out pdf blank, edit, and sign them. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical. Web complete medwatch form online with us legal forms. For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web online using the medwatch online reporting form; Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch:
Web complete medwatch form online with us legal forms. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical. Web form fda 3500 author: Web online using the medwatch online reporting form; Web a copy of a dhmh medwatch form and instructions are available at the links below. Easily fill out pdf blank, edit, and sign them. Save or instantly send your ready documents. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form.
FDA MedWatch Pioglitazonecontaining Medicines Drug Safety
Web complete medwatch form online with us legal forms. Easily fill out pdf blank, edit, and sign them. Web a copy of a dhmh medwatch form and instructions are available at the links below. Patient or patient healthcare representative: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health.
fda form 3500a Fill out & sign online DocHub
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,.
MedWatchLogo1 KnowYourOTCS
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web online using the medwatch online reporting form; Web a copy of a dhmh medwatch form and instructions are available at the links below. Save or instantly send your ready documents. Web medwatch.
Medwatch Instructions For Medwatch Form 3500 Voluntary Reporting Of
Patient or patient healthcare representative: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Easily fill out pdf blank, edit, and sign them. Web complete medwatch form online with us legal forms. For voluntary reporting of adverse events, product problems and product.
3 Medwatch Form Templates free to download in PDF
Web a copy of a dhmh medwatch form and instructions are available at the links below. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web complete medwatch form online with us legal forms. For voluntary reporting of adverse events, product problems and product use/medication errors created date: Save or instantly send your ready.
Regulatory Submissions Product Documentation
Easily fill out pdf blank, edit, and sign them. Patient or patient healthcare representative: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web online using the medwatch online reporting form; Safety alerts for human medical products (drugs, biologics.
Form FDA 3500B MEDWATCH Consumer Voluntary Reporting Free Download
Web a copy of a dhmh medwatch form and instructions are available at the links below. Web form fda 3500 author: Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web complete medwatch form online with us legal forms. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product.
The Importance of HighQuality Reporting of an SAE Case Report The
Easily fill out pdf blank, edit, and sign them. Safety alerts for human medical products (drugs, biologics. Web online using the medwatch online reporting form; Save or instantly send your ready documents. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch:
Fda Medwatch Form Fill Out and Sign Printable PDF Template signNow
Web form fda 3500 author: Web online using the medwatch online reporting form; Web a copy of a dhmh medwatch form and instructions are available at the links below. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Save or instantly send your ready documents.
MedWatch Forms YouTube
For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Save or instantly send your ready documents. Web medwatch is the food and drug administration's (fda) program for reporting.
Web Complete Medwatch Form Online With Us Legal Forms.
Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical. Web online using the medwatch online reporting form; Easily fill out pdf blank, edit, and sign them. Patient or patient healthcare representative:
Web A Copy Of A Dhmh Medwatch Form And Instructions Are Available At The Links Below.
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Safety alerts for human medical products (drugs, biologics. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch:
For Voluntary Reporting Of Adverse Events, Product Problems And Product Use/Medication Errors Created Date:
Web form fda 3500 author: Save or instantly send your ready documents.