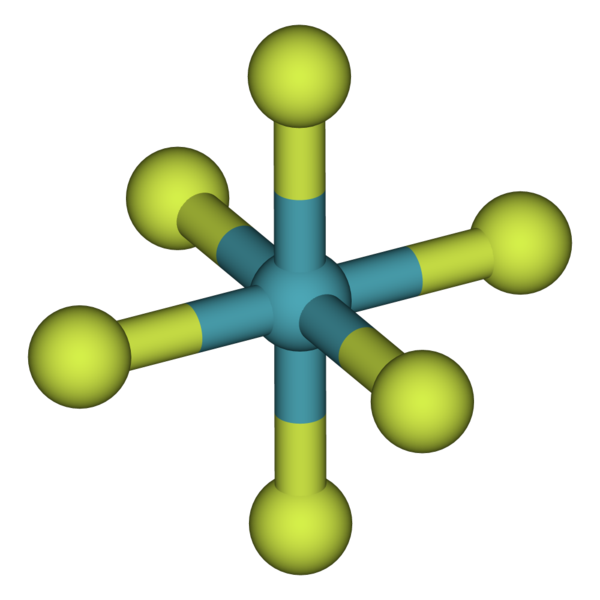
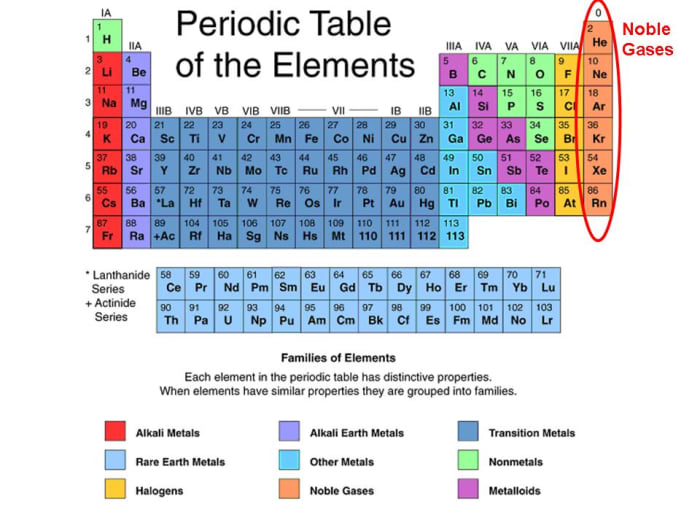
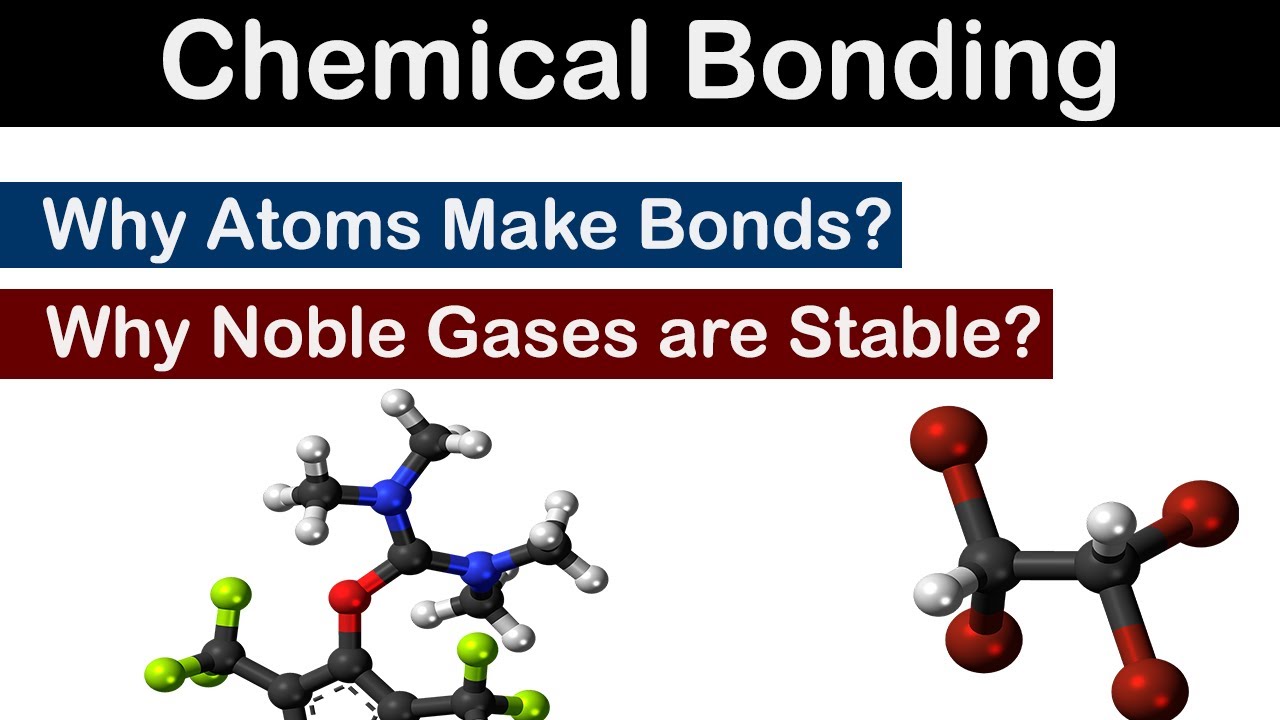
Why Do Noble Gases Rarely Form Bonds With Other Atoms
Why Do Noble Gases Rarely Form Bonds With Other Atoms - Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. Web colors of noble gases. Web why are the noble gases called noble? Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web the noble gases rarely form compounds. Many of these gases are used in displays because of their. The ability to avoid reacting when provoked—to turn up one's nose and ignore lesser human foibles—is largely considered. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Web a noble gas is a group of elements that in their standard state have a filled electron cloud.
The ability to avoid reacting when provoked—to turn up one's nose and ignore lesser human foibles—is largely considered. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. The different gases glow when an electric current is passed through them. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. Many of these gases are used in displays because of their. Although the noble gases are generally. Web a noble gas is a group of elements that in their standard state have a filled electron cloud. Since they have full outer. Web why are the noble gases called noble?
Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the matrix of. (apex) why do noble gases rarely form bonds with other atoms a the noble gases are not reactive, so they don't need full valence. The elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely form chemical bonds. Since they have full outer. The ability to avoid reacting when provoked—to turn up one's nose and ignore lesser human foibles—is largely considered. Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons. Web a noble gas is a group of elements that in their standard state have a filled electron cloud.
The groups, and electron dot diagrams Presentation Chemistry
(apex) why do noble gases rarely form bonds with other atoms a the noble gases are not reactive, so they don't need full valence. Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the matrix of. Web colors of noble gases. Web noble gases have a.
Group 18 The Noble Gases
Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. Since they have full outer. Web under ordinary conditions, noble gases are inert and don't form compounds,.
Why do noble gases have larger atomic size ? YouTube
Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. These elements are found in the 18th column of the periodic table and include helium. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of.
What Is The Reactivity Of Noble Gases howtogetalaid
Web colors of noble gases. The elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely form chemical bonds. Since they have full outer. Although the noble gases are generally. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their.
Why do Noble Gases rarely form Bonds with other Atoms? MakeTheBrainHappy
Web colors of noble gases. Web the noble gases rarely form compounds. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. The ability to avoid reacting when provoked—to turn up one's nose and ignore lesser human foibles—is largely considered. They have the most stable configuration (full octet,.
What's So Noble About Noble Gases? Owlcation Education
Many of these gases are used in displays because of their. Web the noble gases rarely form compounds. Web answered • expert verified. This graphic summarizes this quite well. The elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely form chemical bonds.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?
This graphic summarizes this quite well. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their. Since they have full outer. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. Web colors of noble gases.
What Are Noble Gases? Definition and Properties
Although the noble gases are generally. Web answered • expert verified. Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. They have the most stable configuration.
Why do noble gases have large atomic radii Part 47P blockUnit 7I
Web the noble gases rarely form compounds. Web the noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules h 2, n. Web answered • expert verified. The elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely form chemical bonds. Web.
Why Atoms Make Bonds Why Noble Gases are Stable Chemical Bonding
Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the matrix of. Since they have full outer. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. This graphic summarizes this quite well. Web.
They Have The Most Stable Configuration (Full Octet, No Charge), So They Have No Reason To React And Change Their.
Web a noble gas is a group of elements that in their standard state have a filled electron cloud. Although the noble gases are generally. Web atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons. Web in chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table.
The Different Gases Glow When An Electric Current Is Passed Through Them.
Web the noble gases rarely form compounds. Web answered • expert verified. Web overall, noble gases have weak interatomic forces, and therefore very low boiling and melting points compared with elements of other groups. Since they have full outer.
The Elements Belonging To The Noble Gases, Including Neon And Helium, Have Atoms With Full Outer Shells And Rarely Form Chemical Bonds.
The ability to avoid reacting when provoked—to turn up one's nose and ignore lesser human foibles—is largely considered. Web why are the noble gases called noble? Web under ordinary conditions, noble gases are inert and don't form compounds, but when ionized or under pressure, they will sometimes working into the matrix of. These elements are found in the 18th column of the periodic table and include helium.
Web The Noble Gases Are All Monatomic, Whereas The Other Nonmetal Gases—Hydrogen, Nitrogen, Oxygen, Fluorine, And Chlorine—Normally Exist As The Diatomic Molecules H 2, N.
Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. (apex) why do noble gases rarely form bonds with other atoms a the noble gases are not reactive, so they don't need full valence. Many of these gases are used in displays because of their. Web colors of noble gases.
.PNG)
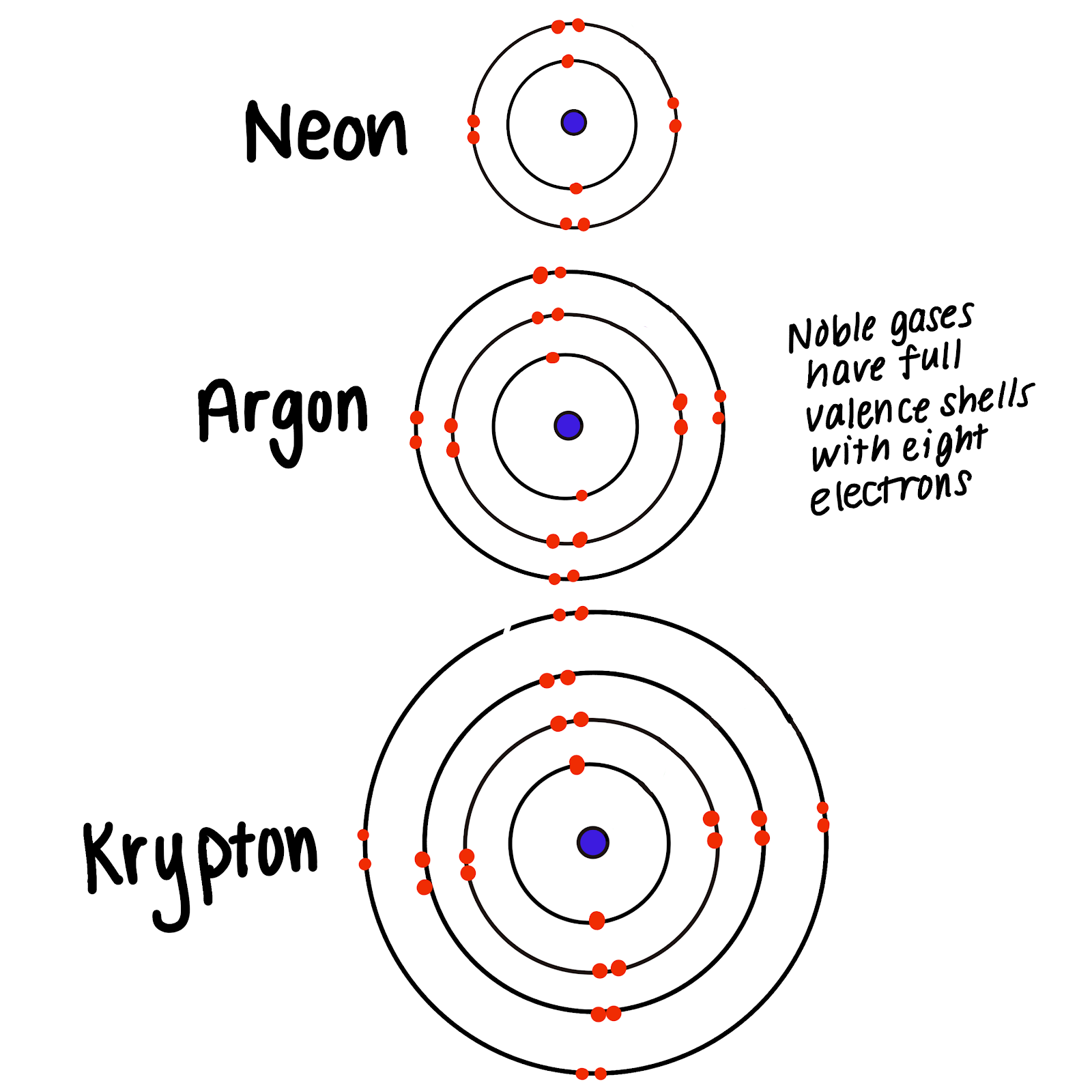

.PNG)