States Of Matter Chapter 10 Review
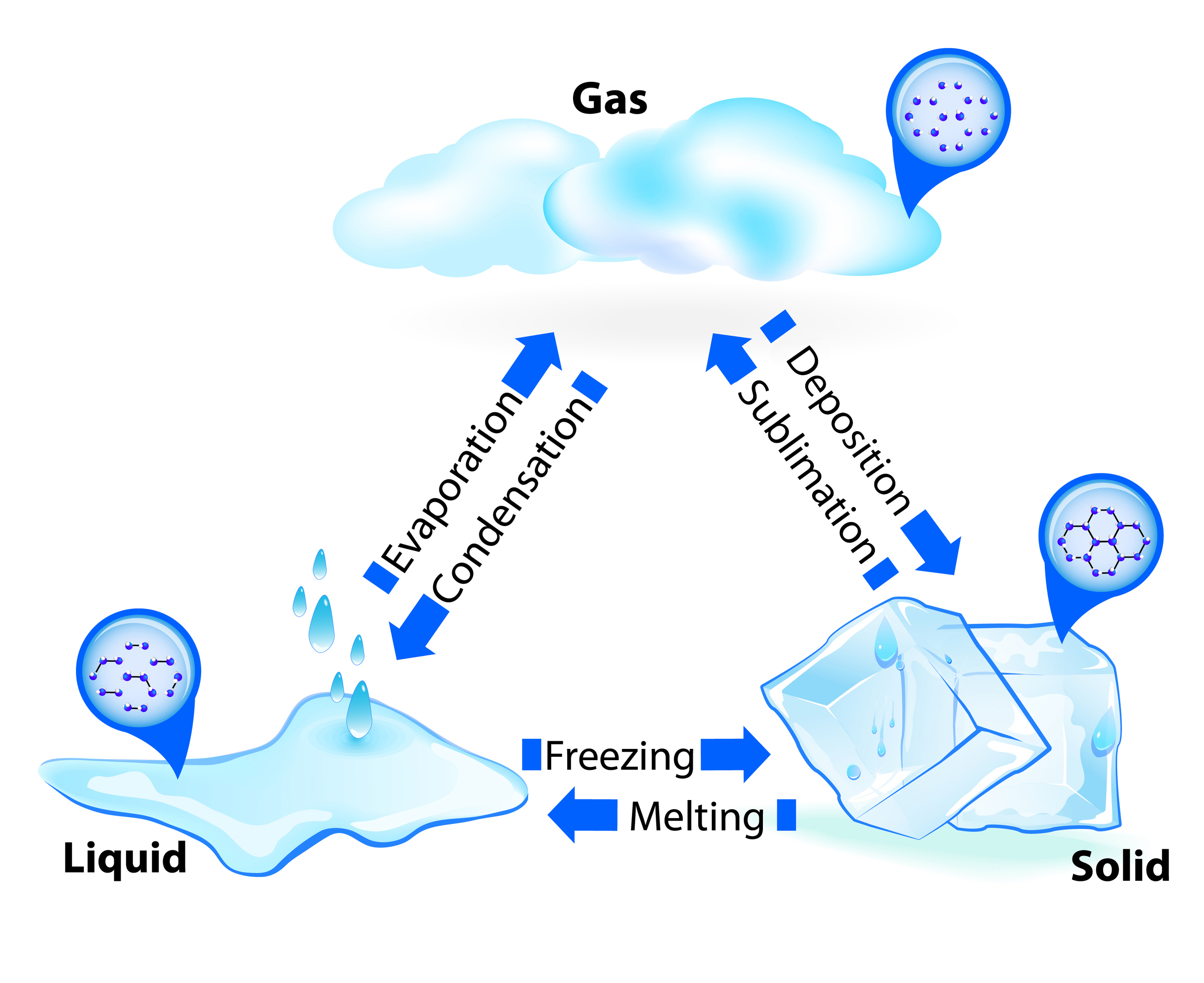
States Of Matter Chapter 10 Review - Chapter 10 states of matter test answers. The heart of the matter. Web chapter 10 review states of matter section 1 short answeranswer the following questions in the space provided. Physical property of matter that indicates weather a sample of matter is a solid, liquid, gas, or plasma. How does the motion of particles change as a substance. Find other quizzes for chemistry and more on quizizz for free! Which one of these could be described as having high density and a definite volume? Click the card to flip 👆. Web what state of matter is segment 5? Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter.
Click the card to flip 👆. Physical property of matter that indicates weather a sample of matter is a solid, liquid, gas, or plasma. Chem chapter 10 review draft. (d) the ability to change to a gas. Web section review worksheets answer key chapter 10 review worksheet answer key states of matter pp phase changes pp 1 of 5 stars 2 of 5 stars 3 of 5 stars 4 of 5 stars 5 of 5 stars. Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. A state of matter with no definite shape or volume. Find other quizzes for chemistry and more on quizizz for free! Web what state of matter is segment 5?
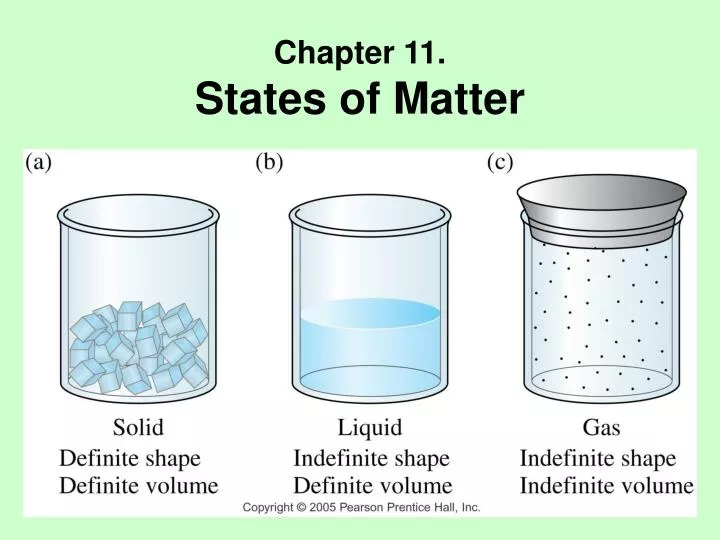
Assume that a, b, c, d, and e are autonomous systems (ass). Web chapter 10 review states of matter section 1 short answeranswer the following questions in the space provided. 1 of 5 stars 2 of 5 stars 3 of 5 stars 4 of 5 stars 5 of 5 stars. Chapter 10 states of matter test answers. Find the path vector for each as using the algorithm in table 20.3. Solid liquid gas definite volume definite volume indefinite volume definite shape indefinite shape indefinite shape strong intermolecular (attractive) forces moderately strong. Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Download chapter 10 states of matter test answers: States of matter quiz for 9th grade students. Web comparing the states of matter;
Chapter 3 States of Matter
Assume that a, b, c, d, and e are autonomous systems (ass). States of matter quiz for 9th grade students. Web holt mcdougal modern chemistry 1 states of matter chapter 10 review states of matter section 2 short answer answer the following questions in the space provided. 3.97 avg rating — 28,463 ratings. Web section review worksheets answer key chapter.
States of Matter
Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Solid liquid gas definite volume definite volume indefinite volume definite shape indefinite shape indefinite shape strong intermolecular (attractive) forces moderately strong. 99 ,.
PPT Chapter 10 States of Matter PowerPoint Presentation, free
Web learn about the definition of the kinetic theory of matter, phase changes, and the four states of matter. Find the path vector for each as using the algorithm in table 20.3. States of matter quiz for 9th grade students. The heart of the matter. How does the motion of particles change as a substance.
PPT Chapter 10 States of Matter PowerPoint Presentation, free
Web learn about the definition of the kinetic theory of matter, phase changes, and the four states of matter. Define solid, liquid, and gas. _____ liquids possess all the following. Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Click the card to flip 👆.
States of Matter
How does the motion of particles change as a substance. Define solid, liquid, and gas. Web chapter 10 review states of matter (section 2) 5.0 (4 reviews) liquids possess all the following properties except: Chem chapter 10 review draft. Click let’s review to review.
PPT Chapter 10 Review States of Matter PowerPoint Presentation
Compare and contrast the proximities and mobilities of solid, liquid, and gaseous particles. Download chapter 10 states of matter test answers: Find the path vector for each as using the algorithm in table 20.3. States of matter quiz for 9th grade students. (b) the ability to diffuse.
Chapter 10 states of matter
Relatively low density ionic crystal hard brittle and non conducting covalent molecular crystal has strong covalent bonds between neighboring atoms metallic crystal mobile electrons in the crystal covalent network crystal typically has the lowest melting point of. Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter. Solid liquid gas.
PPT Chapter 11. States of Matter PowerPoint Presentation, free
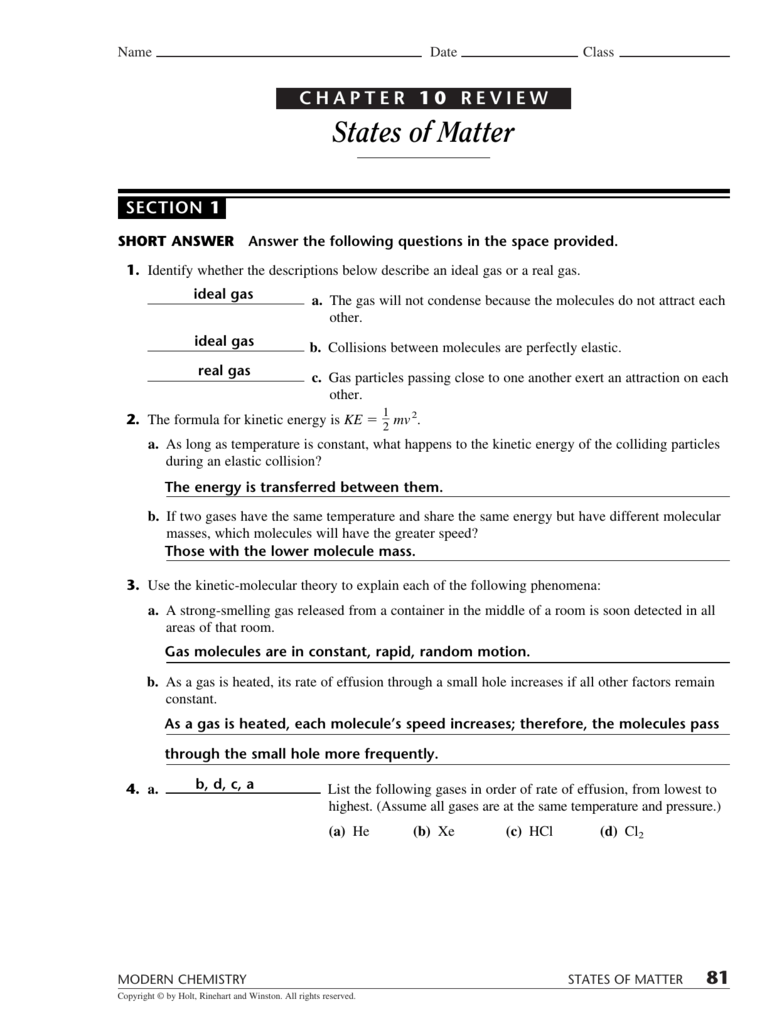
A state of matter with no definite shape or volume. States of matter quiz for 9th grade students. 3.97 avg rating — 28,463 ratings. Web chapter 10 review states of matter (section 1) 4.3 (9 reviews) the gas will not condense because the molecules do not attract each other. Web chapter 10 review states of matter section 1 short answeranswer.
Wild Adventures October 2013
Download chapter 10 states of matter test answers: Click the card to flip 👆. Define solid, liquid, and gas. 99 , and 1 person voted. Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter.
Unit 2 Review States of Matter Crossword WordMint
Click the card to flip 👆. States of matter quiz for 9th grade students. Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter. Web chapter 10 review states of matter (section 2) 5.0 (4 reviews) liquids possess all the following properties except: Based on idea that particles of matter.
Web Holt Mcdougal Modern Chemistry 1 States Of Matter Chapter 10 Review States Of Matter Section 2 Short Answer Answer The Following Questions In The Space Provided.
Download chapter 10 states of matter test answers: Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Physical property of matter that indicates weather a sample of matter is a solid, liquid, gas, or plasma. Chem chapter 10 review draft.
Ideal Gas Molecules Do Not Repel Or.
Matter with a definite shape and volume. Click the card to flip 👆. (d) the ability to change to a gas. Matter that has a definite volume but no definite shape.
Relatively Low Density Ionic Crystal Hard Brittle And Non Conducting Covalent Molecular Crystal Has Strong Covalent Bonds Between Neighboring Atoms Metallic Crystal Mobile Electrons In The Crystal Covalent Network Crystal Typically Has The Lowest Melting Point Of.
Chapter 10 states of matter test answers. Web chapter 10 review states of matter section 1 short answeranswer the following questions in the space provided. Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter. 99 , and 1 person voted.
Assume That A, B, C, D, And E Are Autonomous Systems (Ass).
1 of 5 stars 2 of 5 stars 3 of 5 stars 4 of 5 stars 5 of 5 stars. Find the path vector for each as using the algorithm in table 20.3. How does the motion of particles change as a substance. _____ liquids possess all the following.