Metals Form What Type Of Ions
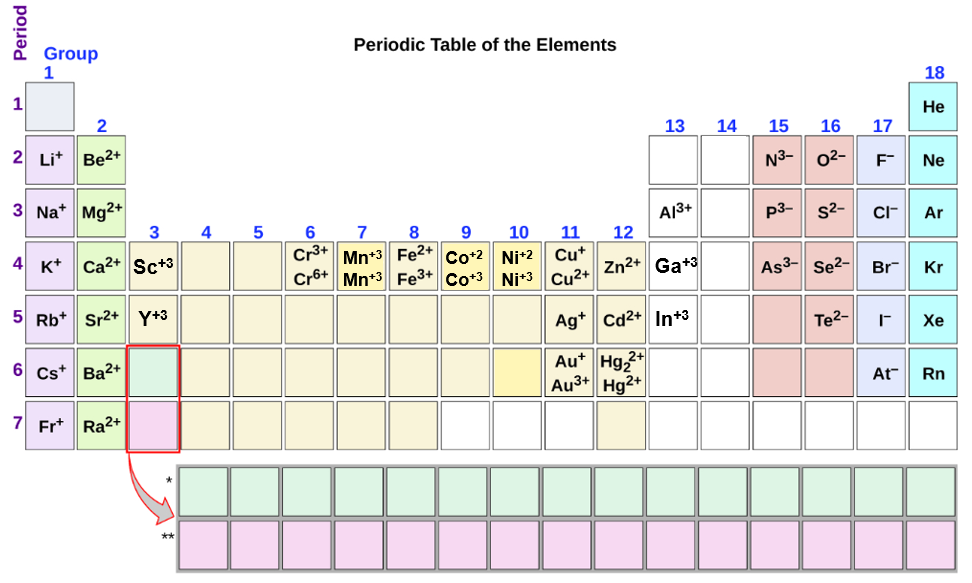
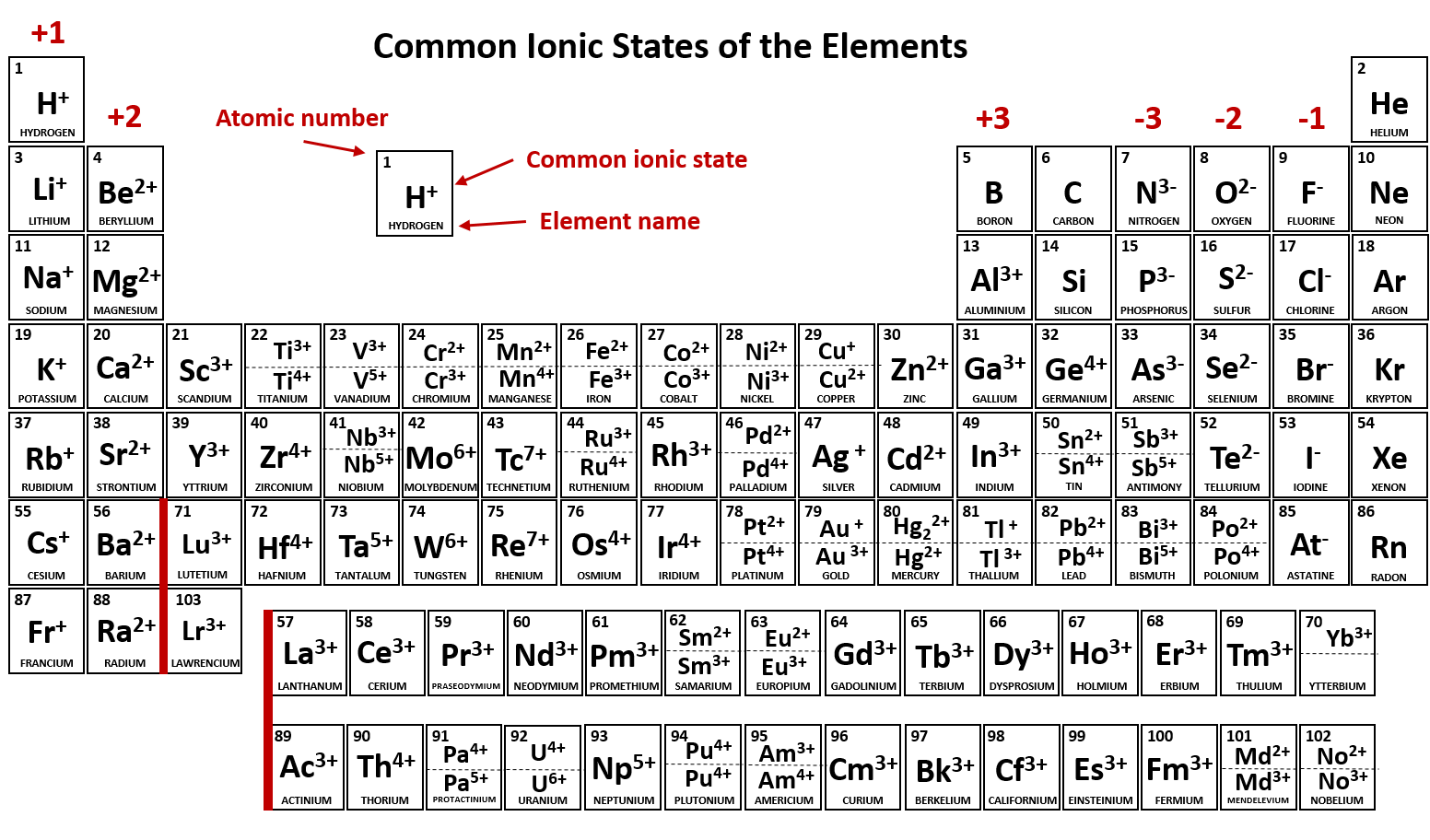
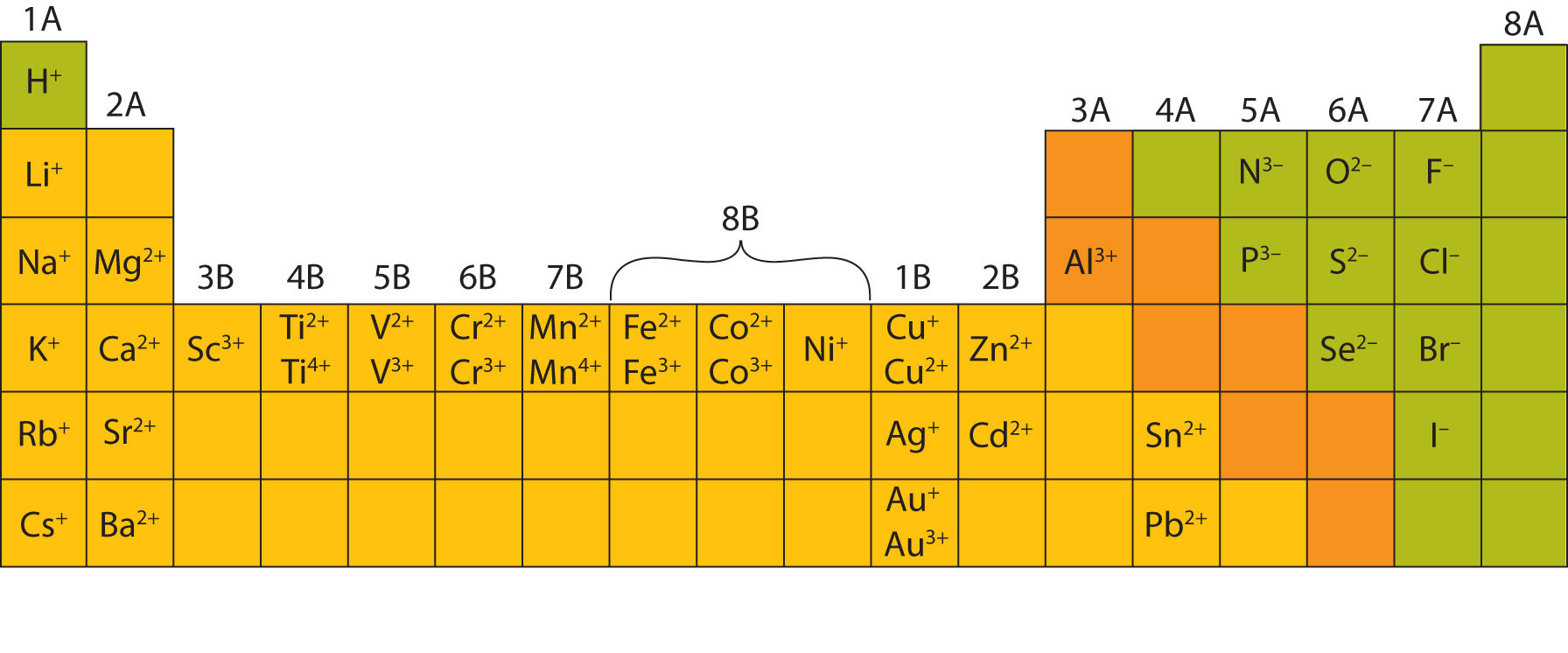
Metals Form What Type Of Ions - Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Metal atoms lose electrons to. In these compounds you have the cation, often an alkali metal itself, stabilized in. The solvation number , n , determined by a variety of. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or can have multiple numbers of electrons they. Web cations of metals that form only one type of ion. Web group 13 elements form +3 ions. Predicting the type of bonding in compounds potassium (group 1) is a metal, and iodine (group 17) is a nonmetal; Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. The charge of an electron is considered to be negative by convention and this charge is equal and.
The solvation number , n , determined by a variety of. The charge of an electron is considered to be negative by convention and this charge is equal and. Ki is predicted to be ionic. Metals tend to form cations, while nonmetals tend to form anions. Web group iv a metals form cations with a +4 charge, whereas tin (sn) and lead (pb) can form cations with a +2 charge. Web answer:metals form positive ions, or cations.atoms achieve this type of configuration by gaining or losing electrons depending on the number of electrons in their metals usually. Most nonmetals become anions when they make ionic compounds. In these compounds you have the cation, often an alkali metal itself, stabilized in. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. Second, most atoms form ions of a.
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Ki is predicted to be ionic. Web group 13 elements form +3 ions. The solvation number , n , determined by a variety of. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web answer:metals form positive ions, or cations.atoms achieve this type of configuration by gaining or losing electrons depending on the number of electrons in their metals usually. The charge of an electron is considered to be negative by convention and this charge is equal and. Web negatively charged ions are called anions. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. When atoms of nonmetal elements form ions, they.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Web group 13 elements form +3 ions. Ions form when atoms lose or gain electrons to obtain a full outer shell: Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. Web ion, any atom or group of atoms that bears.
PPT 1 Name the ions formed by these elements and classify them as
Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2+ is called a calcium ion. Carbon (c) and silicon (si) are nonmetals that rarely form. Web cations of metals that form only one type of ion. The charge of an electron is considered to be negative.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Web cations of metals that form only one type of ion. Metal ions with low oxidation. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. In these compounds you have the cation, often an alkali metal itself, stabilized in. Web group 13 elements form +3 ions.
Colours of Transition Metal Ions in Aqueous Solution [Infographic
The names for positive and negative ions are. The charge of an electron is considered to be negative by convention and this charge is equal and. Carbon (c) and silicon (si) are nonmetals that rarely form. Web up to $3 cash back metal ions with high oxidation numbers form stronger bonds to ligands than metal ions with low oxidation numbers..
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
The charge of an electron is considered to be negative by convention and this charge is equal and. Web negatively charged ions are called anions. The names for positive and negative ions are. Click the card to flip 👆. Web cations of metals that form only one type of ion.
Naming Simple Ionic Compounds Pathways to Chemistry
Click the card to flip 👆. Web this is actually one of the chemical properties of metals and nonmetals: Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web up to $3 cash back metal ions with high oxidation numbers form stronger bonds to ligands.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web this is actually one of the chemical properties of metals and nonmetals: Web group iv a metals form cations with a +4 charge, whereas tin (sn) and lead (pb) can form cations with a +2 charge. Web what type of ions do metals form? Ki is predicted to be ionic. Web group 13 elements form +3 ions.
Chem matters ch6_ionic_bond
Web cations of metals that form only one type of ion. The charge of an electron is considered to be negative by convention and this charge is equal and. Web up to $3 cash back metal ions with high oxidation numbers form stronger bonds to ligands than metal ions with low oxidation numbers. The names for positive and negative ions.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web answer:metals form positive ions, or cations.atoms achieve this type of configuration by gaining or losing electrons depending on the number of electrons in their metals usually. Web what type of ions do metals form? Web an ion is an atom or group of atoms with a positive or negative charge. Carbon (c) and silicon (si) are nonmetals that rarely.
In These Compounds You Have The Cation, Often An Alkali Metal Itself, Stabilized In.
Group 14 elements form none. Click the card to flip 👆. Ki is predicted to be ionic. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or can have multiple numbers of electrons they.
Web Group Iv A Metals Form Cations With A +4 Charge, Whereas Tin (Sn) And Lead (Pb) Can Form Cations With A +2 Charge.
Most nonmetals become anions when they make ionic compounds. Metals tend to form cations, while nonmetals tend to form anions. The names for positive and negative ions are. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+.
The Charge Of An Electron Is Considered To Be Negative By Convention And This Charge Is Equal And.
Web answer:metals form positive ions, or cations.atoms achieve this type of configuration by gaining or losing electrons depending on the number of electrons in their metals usually. Web up to $3 cash back metal ions with high oxidation numbers form stronger bonds to ligands than metal ions with low oxidation numbers. Carbon (c) and silicon (si) are nonmetals that rarely form. Metal ions with low oxidation.
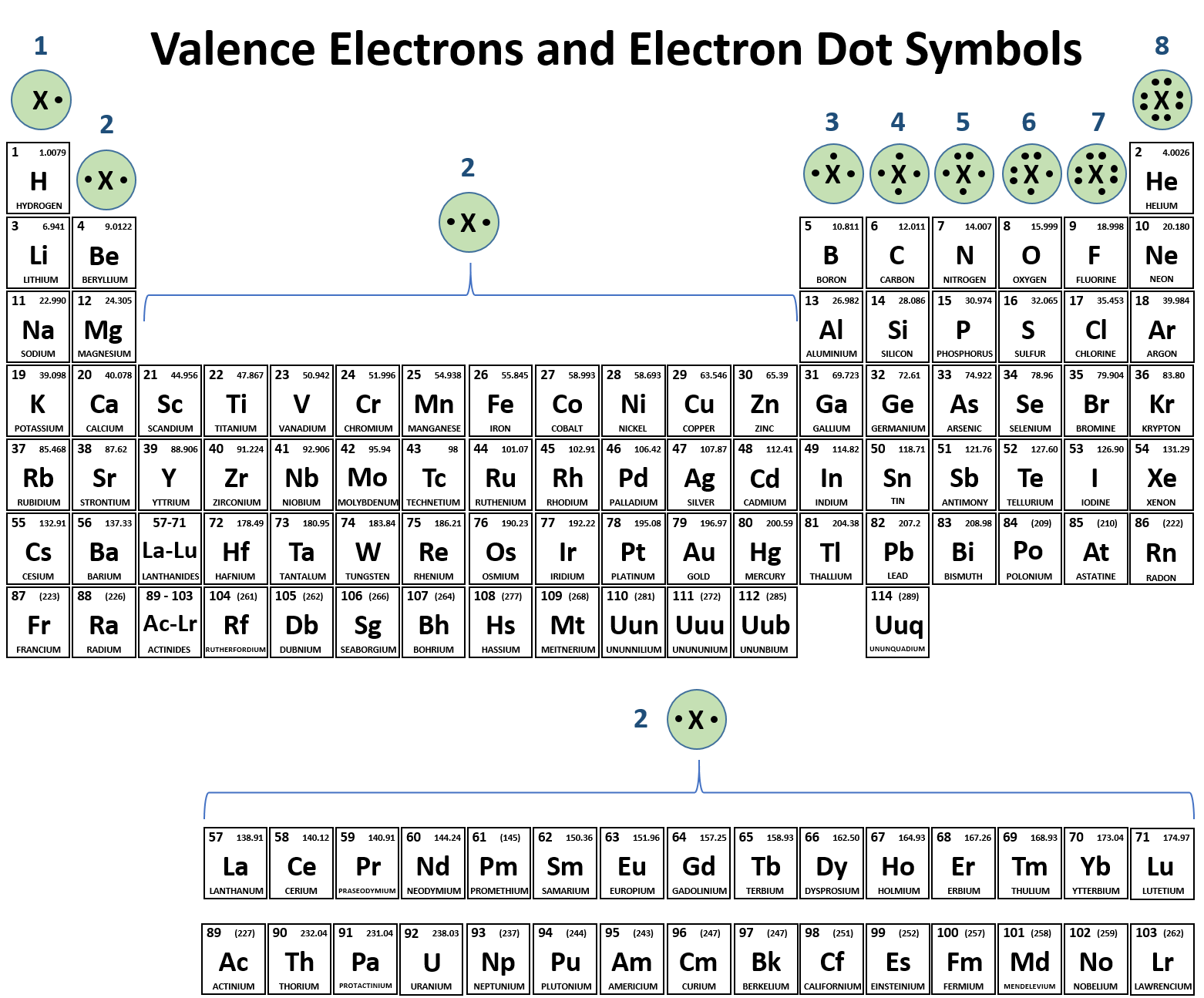
Ions Form When Atoms Lose Or Gain Electrons To Obtain A Full Outer Shell:
Web group 13 elements form +3 ions. Positively charged ions are called cations; Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and activation, nitrogen fixation, and antioxidant. Web this is actually one of the chemical properties of metals and nonmetals: