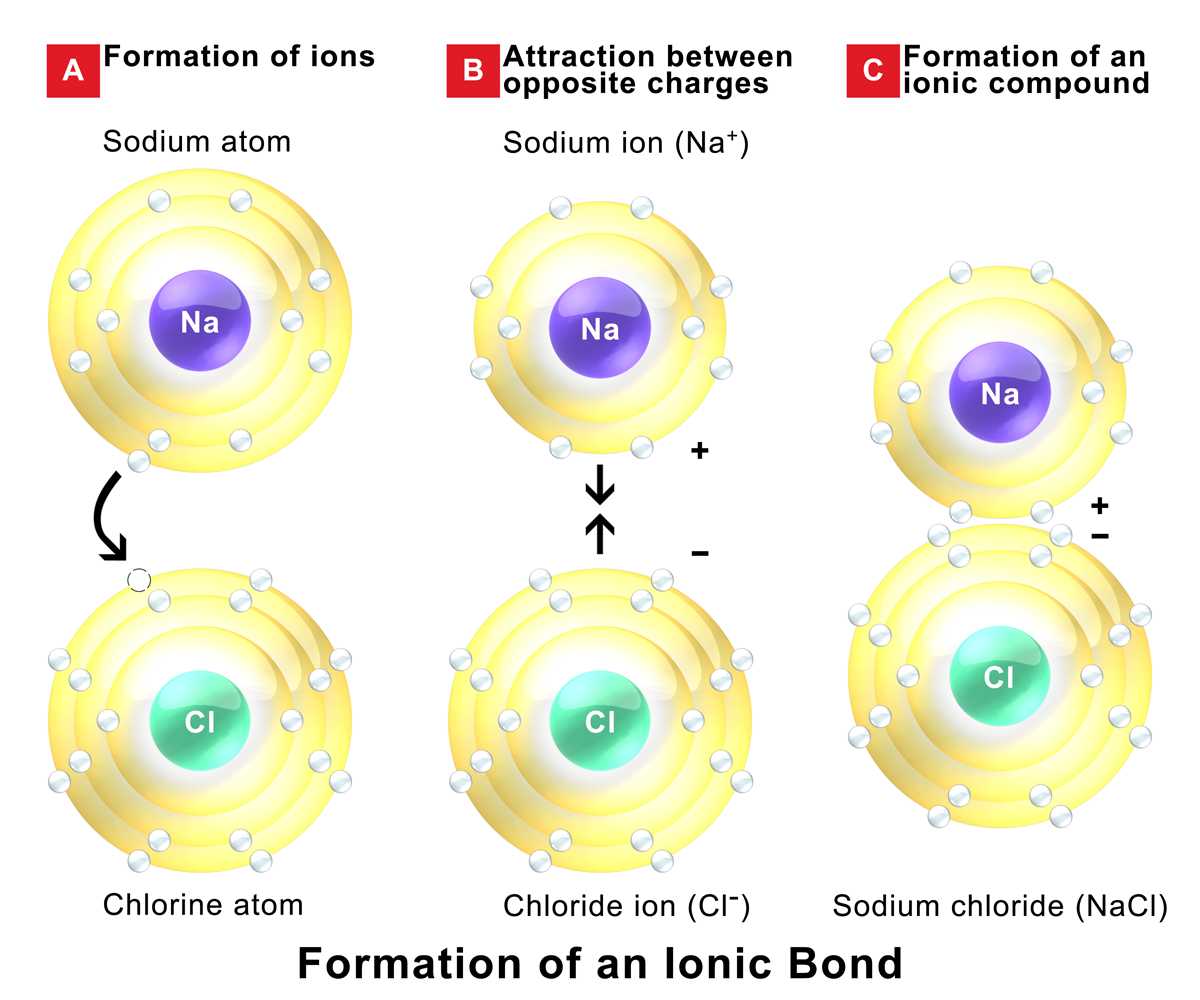
Ionic Bonds Form Between What Types Of Elements
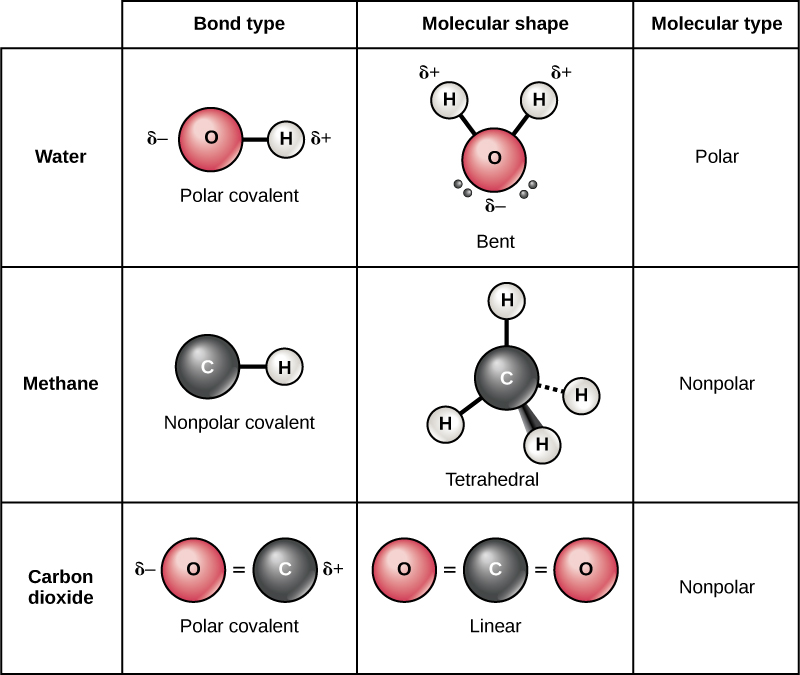
Ionic Bonds Form Between What Types Of Elements - Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Web the most common types of elements that form ionic bonds are metals and nonmetals. Charged chemical species form when neutral atoms, or groups of atoms, lose. Because the number of electrons does not equal the number of protons, each ion has a. Web when an atom does not contain equal numbers of protons and electrons, it is called an ion. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Ionic bonds are formed between a cation, which. Web about 30% of respondents selected all three correctly. Web an ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions.
Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). One of the atoms (metal) involved in the bond formation must have a low. Web one type of chemical bond is an ionic bond. Ionic bonds are formed between a cation, which. Web when an atom does not contain equal numbers of protons and electrons, it is called an ion. For example, sodium cations (positively charged ions). Electron transfer produces negative ions called anions and positive ions. Web an ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Web 1 point if the element lithium (li) were to bond with the element sulfur (s), what type of bond can you predict will be formed and why?* a.
Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Web an ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Web one type of chemical bond is an ionic bond. Charged chemical species form when neutral atoms, or groups of atoms, lose. Different types of bonds form. Web when an atom does not contain equal numbers of protons and electrons, it is called an ion. Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Electron transfer produces negative ions called anions and positive ions. One of the atoms (metal) involved in the bond formation must have a low. Ionic bonds result from the attraction between oppositely charged ions.
Ionic Bond Definition, Types, Properties & Examples
Web about 30% of respondents selected all three correctly. One of the atoms (metal) involved in the bond formation must have a low. Web ionic bonds are one of the two main types of chemical bonds. A number of students could not “…discriminate between particulate representations of compounds and elements” (p. Return to bonding menu in modern language, the central.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
One of the atoms (metal) involved in the bond formation must have a low. For example, sodium cations (positively charged ions). Electron transfer produces negative ions called anions and positive ions. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web the most common types of elements that form ionic.
Ionic Bond Definition, Types, Properties & Examples
Charged chemical species form when neutral atoms, or groups of atoms, lose. Web about 30% of respondents selected all three correctly. Web 1 point if the element lithium (li) were to bond with the element sulfur (s), what type of bond can you predict will be formed and why?* a. Web which elements form ionic bonds? They form as a.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Because the number of electrons does not equal the number of protons, each ion has a. Web which elements form ionic bonds? Ionic bonds result from the attraction between oppositely charged ions. Transfer of the electrons is energetically. Web about 30% of respondents selected all three correctly.
Examples of Ionic Bonds and Ionic Compounds
Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Web which elements form ionic bonds? A number of students could not “…discriminate between particulate representations of compounds and elements” (p. One of the atoms (metal) involved in the bond formation must have a low. Transfer of the electrons is energetically.
Examples of Ionic Bonding YouTube
For example, sodium cations (positively charged ions). Web the most common types of elements that form ionic bonds are metals and nonmetals. Electron transfer produces negative ions called anions and positive ions. Because the number of electrons does not equal the number of protons, each ion has a. Ionic bonds result from the attraction between oppositely charged ions.
Ionic Properties
Ionic bonds result from the attraction between oppositely charged ions. Web ionic bonds are one of the two main types of chemical bonds. One of the atoms (metal) involved in the bond formation must have a low. Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Different types of bonds form.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
A number of students could not “…discriminate between particulate representations of compounds and elements” (p. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web which elements form ionic bonds? Different types of bonds form. Web ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web which elements form ionic bonds? Different types of bonds form. Electron transfer produces negative ions called anions and positive ions. For example, sodium cations (positively charged ions). Web 1 point if the element lithium (li) were to bond with the element sulfur (s), what type of bond can you predict will be formed and why?* a.
Ionic Bond Definition, Types, Properties & Examples
Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. For example, sodium cations (positively charged ions). Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Return to bonding menu in modern language, the central idea of an ionic bond is that.
Web One Type Of Chemical Bond Is An Ionic Bond.
Ionic bonds are formed between a cation, which. Transfer of the electrons is energetically. For example, sodium cations (positively charged ions). Web 1 point if the element lithium (li) were to bond with the element sulfur (s), what type of bond can you predict will be formed and why?* a.
Charged Chemical Species Form When Neutral Atoms, Or Groups Of Atoms, Lose.
Web an ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Web ionic bonds are one of the two main types of chemical bonds. Web ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion.
Different Types Of Bonds Form.
They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web the most common types of elements that form ionic bonds are metals and nonmetals. A number of students could not “…discriminate between particulate representations of compounds and elements” (p. Because the number of electrons does not equal the number of protons, each ion has a.
Web Ionic Bonds Form When A Nonmetal And A Metal Exchange Electrons, While Covalent Bonds Form When Electrons Are Shared Between Two Nonmetals.
Web when an atom does not contain equal numbers of protons and electrons, it is called an ion. One of the atoms (metal) involved in the bond formation must have a low. Web ionic bonds form between elements with very different electronegativities, resulting in transfer of electrons between the atoms. Web which elements form ionic bonds?


.PNG)
:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)