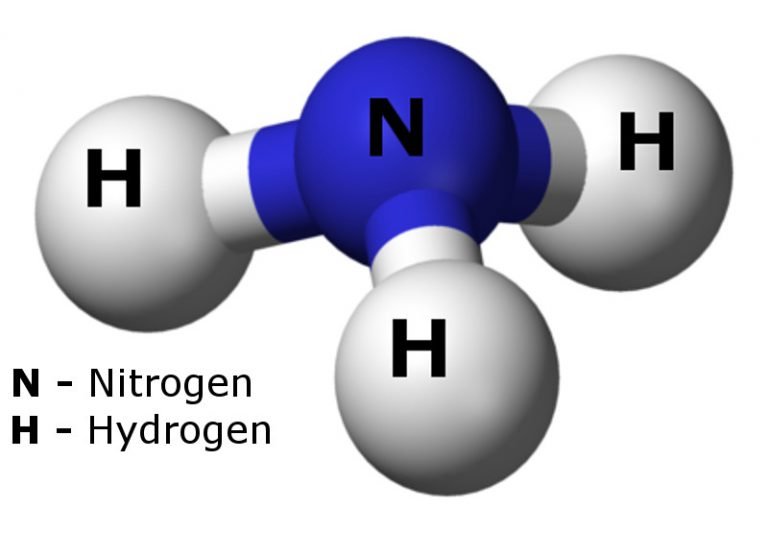
Hydrogen And Nitrogen Combine To Form Ammonia
Hydrogen And Nitrogen Combine To Form Ammonia - The mass of nitrogen and hydrogen which combine together to form 6.8 g ammonia is. Write a balanced chemical equation for this reaction? Hydrogen and nitrogen combine to form ammonia. The mechanism of ammonia synthesis contains the following seven elementary steps: Pore diffusion to the reaction center Calculate (in kj) the standard enthalpy change h for the reaction written below, using the bond energies. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction. If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. I mean to say that, when hydrogen (g) and nitrogen (g) combines together.
Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Web nitrogen gas and hydrogen gas combine to produce ammonia gas. Write a balanced chemical equation for this reaction? H 2 hydrogen + n 2 nittrogen → nh 3. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; I mean to say that, when hydrogen (g) and nitrogen (g) combines together. If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. The mass of nitrogen and hydrogen which combine together to form 6.8 g ammonia is. Hydrogen and nitrogen combine to form ammonia.
Web nitrogen gas and hydrogen gas combine to form ammonia (nh3) gas. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web nitrogen and hydrogen combine to form ammonia in the haber process. Web hydrogen and nitrogen combine in a synthesis reaction to form ammonia (nh 3) in the haber process. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). I mean to say that, when hydrogen (g) and nitrogen (g) combines together. When nitrogen and hydrogen bond, nitrogen pulls the electrons from hydrogen toward itself. Nitrogen and hydrogen combine to form ammonia in the haber process. Web nitrogen and hydrogen combine to form ammonia which is the only product derived from this reaction. Nitrogen and hydrogen combine to form ammonia in the haber process.
Is Ammonia an Acid or Base? » Science ABC
The mass of nitrogen and hydrogen which combine together to form 6.8 g ammonia is. Web nitrogen and hydrogen combine to form ammonia via the following reaction: 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. The mechanism of ammonia synthesis contains the following seven elementary steps:.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction. Web nitrogen and hydrogen combine to form ammonia which is the only product derived from this reaction. Hydrogen reacts with nitrogen to produce ammonia. Web.
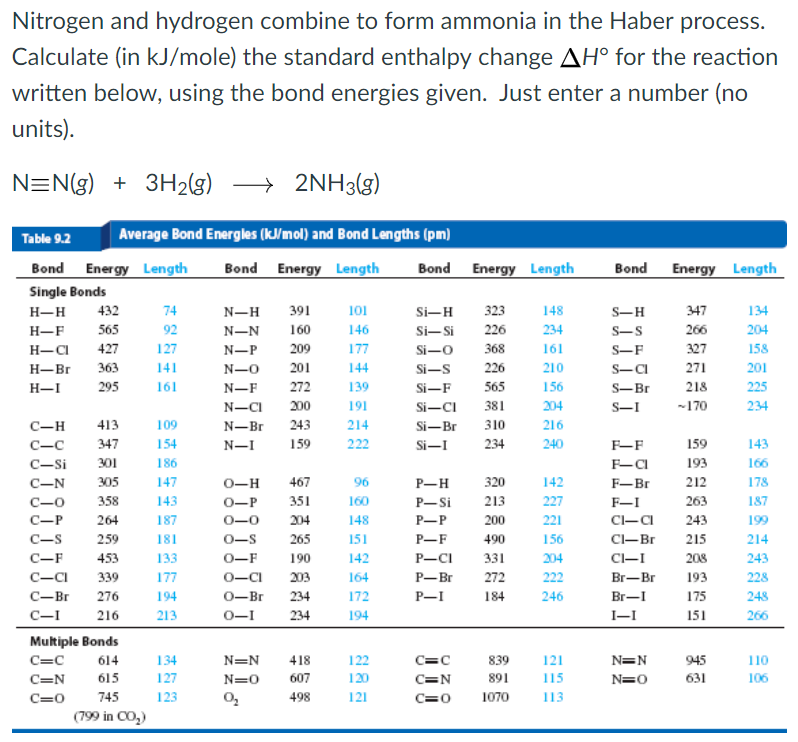
Solved Nitrogen And Hydrogen Combine To Form Ammonia In T...
Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Web nitrogen gas and hydrogen gas combine to form ammonia (nh3) gas. Web hydrogen and nitrogen combine in a synthesis reaction to form ammonia (nh 3) in the haber process. I mean to say that, when hydrogen (g) and nitrogen (g) combines.
Solved Nitrogen and hydrogen combine to form ammonia in the
Representation of the chemical reaction: Web hydrogen and nitrogen combine to form ammonia. Hydrogen and nitrogen combine to form ammonia. Calculate (in kj) the standard enthalpy change h for the reaction written below, using the bond energies. Translate the following into a chemical equation and balance the equation.
Solved Nitrogen and hydrogen react to produce ammonia
Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Calculate (in kj) the standard enthalpy change ah for the reaction written below, using. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Hydrogen and nitrogen combine to form ammonia. The mechanism of ammonia synthesis contains the following seven.
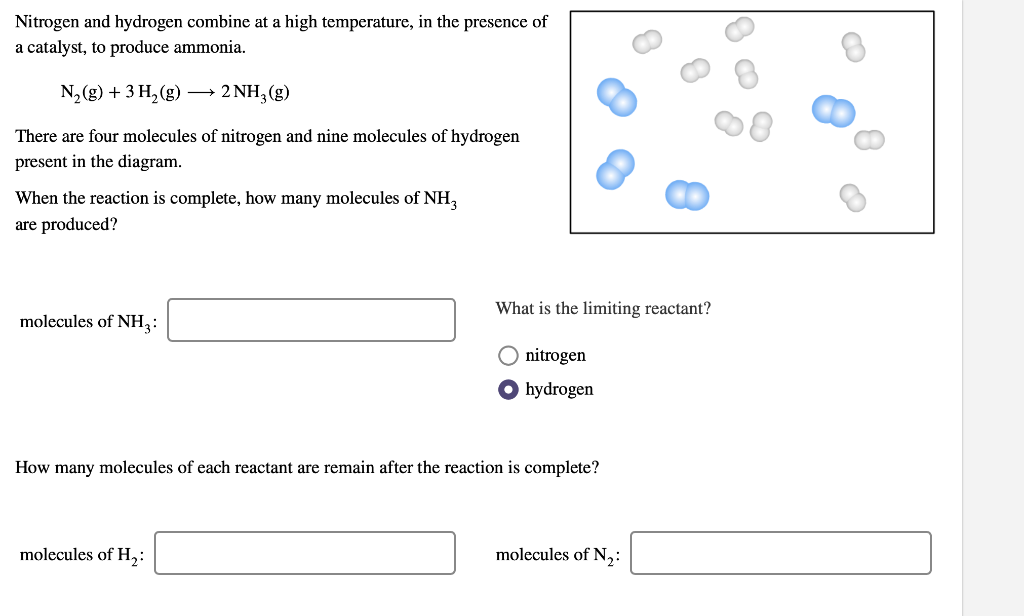
Solved Nitrogen and hydrogen combine at a high temperature,
Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Hydrogen reacts with nitrogen to produce ammonia. N2 +.
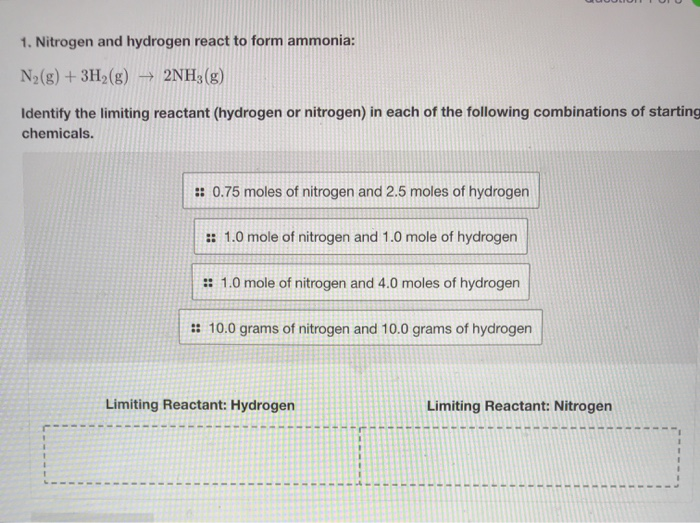
Solved 1. Nitrogen and hydrogen react to form ammonia N2(g)
If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). Web hydrogen gas combines with nitrogen to form ammonia. Translate the following into a chemical equation and balance the equation. Web yes, it is absolutely ture, when hydrogen react with nitrogen.
Solved 10 g of nitrogen is reacted with 5.0 g of hydrogen to
Web hydrogen gas combines with nitrogen to form ammonia. If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. Combination reaction explanation:hydrogen (h2) combines with nitrogen (n2) to form a single compound, ammonia (nh3) in a combination reaction. Web hydrogen and nitrogen combine to form ammonia. Web 1 day agofor the second quarter of 2023, cvr partners’.
Solved Under certain conditions, the substances bromine and
Write a balanced chemical equation for this reaction? The reaction can be written as: Representation of the chemical reaction: Web hydrogen and nitrogen combine to form ammonia. H 2 hydrogen + n 2 nittrogen → nh 3.
Under certain conditions, the substances nitrogen and hydrogen combine
Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Web hydrogen gas combines with nitrogen to form ammonia. Nitrogen and hydrogen combine to form ammonia in the haber process. If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. Hydrogen and nitrogen combine to form ammonia.
Nitrogen And Hydrogen Combine To Form Ammonia In The Haber Process.
Web n2+3h2→2nh3 [relative atomic masses of n=14,h=1 ]. Web hydrogen and nitrogen combine to form ammonia. The reaction can be written as: Web nitrogen and hydrogen combine together to form ammonia.
Ammonia Is Usually Produces When Hydrogen.
N2 + 3h2→ 2nh3 [relative atomic masses of n = 14 u, h = 1 u] the mass of nitrogen and. I mean to say that, when hydrogen (g) and nitrogen (g) combines together. Transport of the reactants from the gas phase through the boundary layer to the surface of the catalyst. Web nitrogen gas and hydrogen gas combine to form ammonia (nh3) gas.
Hydrogen And Nitrogen Combine To Form Ammonia.
If 56.4 g of nitrogen are reacted completely with plenty of hydrogen, what. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). Web 1 day agofor the second quarter of 2023, cvr partners’ average realized gate prices for uan showed a reduction over the prior year, down 43 percent to $316 per ton, and. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia.
Web The Following Chemical Reaction Takes Place When Hydrogen Gas Reacts With Nitrogen To Form Ammonia Which Can Be Shown As;
Nitrogen and hydrogen combine to form ammonia in the haber process. Pore diffusion to the reaction center Translate the following into a chemical equation and balance the equation. Representation of the chemical reaction: