How Many Bonds Does Fluorine Form
How Many Bonds Does Fluorine Form - What type of bonds does fluorine and calcium make?. Web fluorine belongs to halogen family and has seven electrons in its valence shell, it can lose 7 electrons or gain 1 electron to attain stable configuration. In higher level chemistry, it may be possible (like in the. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. So it form 1 covalent bo. Atomic fluorine has 7 valence electrons; The polar nature of the bond means that there is a large. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs. Writing lewis structures and such things, fluorine always forms single bonds.
Two core and seven valence. One bond it has 9 electrons, 2 core and 7 valence. Rather than forming 7 bonds, fluorine. What type of bonds does fluorine and calcium make?. Web how many hydrogen bonds can fluorine make? It has 9 electrons, 2 core and 7 valence. The polar nature of the bond means that there is a large. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. In the case of boron in bf 3, three bonds is the maximum possible because. Web for most purposes, i.e.
Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e…. Web for most purposes, i.e. Web 1 bond a fluorine atom (by itself) has 7 valence electrons. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. In the case of boron in bf 3, three bonds is the maximum possible because. The polar nature of the bond means that there is a large. With these electron configurations, none of these atoms will have any formal charge. Web fluorine belongs to halogen family and has seven electrons in its valence shell, it can lose 7 electrons or gain 1 electron to attain stable configuration. What type of bonds does fluorine and calcium make?.
How Many Valence Electrons Does Fluorine Have doodledesignit
Web for most purposes, i.e. It has 9 electrons, 2 core and 7 valence. Web how many hydrogen bonds can fluorine make? With these electron configurations, none of these atoms will have any formal charge. As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e….
Covalent Bonding (Biology) — Definition & Role Expii
Rather than forming 7 bonds, fluorine. In the case of boron in bf 3, three bonds is the maximum possible because. Web for most purposes, i.e. Web how many hydrogen bonds can fluorine make? With these electron configurations, none of these atoms will have any formal charge.
How Many Valence Electrons Does Fluorine Have doodledesignit
Web 1 bond a fluorine atom (by itself) has 7 valence electrons. Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs. Web describe the important exceptions to the octet rule. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. Web for most purposes,.
How Many Valence Electrons Does Fluorine Have?
Web the elements that receive electrons and form bonds are called anion. Two core and seven valence. Rather than forming 7 bonds, fluorine only forms a. Web a single covalent bond. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character.
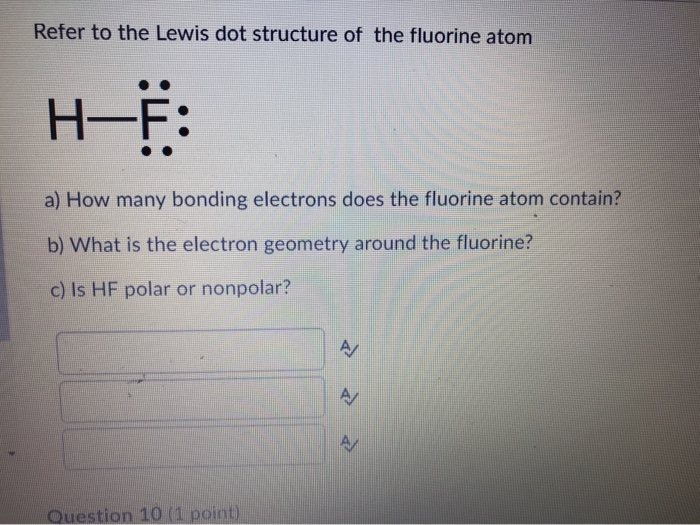
Solved Refer to the Lewis dot structure of the fluorine atom
One bond it has 9 electrons, 2 core and 7 valence. In the case of boron in bf 3, three bonds is the maximum possible because. Web continuing on across the periodic table we see that fluorine is the next element after oxygen. There are six fluorine atoms that form bonds. As a stable electron configuration requires 8 electrons total,.
How Many Bonds Can Magnesium Form TheFitnessManual
Which atoms do not usually form bonds? Two core and seven valence. As a stable electron configuration requires 8 electrons total, fluorine must form 1 bond i.e…. Web a single covalent bond. Web how many hydrogen bonds can fluorine make?
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web a single covalent bond. In the case of boron in bf 3, three bonds is the maximum possible because. It has 9 electrons, 2 core and 7 valence. Rather than forming 7 bonds, fluorine only forms a. Web it follows, therefore, that an atom will tend to make as many covalent bonds as possible.
Periodic Table Fluorine Valence Electrons Periodic Table Timeline
Rather than forming 7 bonds, fluorine. Web how many hydrogen bonds can fluorine make? Web describe the important exceptions to the octet rule. The polar nature of the bond means that there is a large. Based on the electron configuration of fluorine, how many bonds does fluorine form even if it does not undergo hybridization?
氟(一种非金属化学元素)_百度百科
Web 1 bond a fluorine atom (by itself) has 7 valence electrons. Web how many hydrogen bonds can fluorine make? Web continuing on across the periodic table we see that fluorine is the next element after oxygen. With these electron configurations, none of these atoms will have any formal charge. Two core and seven valence.
Fluorine (F) Properties & Uses StudiousGuy
Rather than forming 7 bonds, fluorine. Web how many hydrogen bonds can fluorine make? Based on the electron configuration of fluorine, how many bonds does fluorine form even if it does not undergo hybridization? One bond it has 9 electrons, 2 core and 7 valence. Web for most purposes, i.e.
What Type Of Bonds Does Fluorine And Calcium Make?.
Web continuing on across the periodic table we see that fluorine is the next element after oxygen. Diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. Web fluorine (and all halogens) tends to form one bond and have 3 lone pairs. Web chemistry questions and answers.
Which Atoms Do Not Usually Form Bonds?
Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. The atom would be energetically stable if it achieved an 8 electron count. Rather than forming 7 bonds, fluorine. The polar nature of the bond means that there is a large.
Web How Many Hydrogen Bonds Can Fluorine Make?
In the case of boron in bf 3, three bonds is the maximum possible because. Rather than forming 7 bonds, fluorine only forms a. Web describe the important exceptions to the octet rule. Web fluorine belongs to halogen family and has seven electrons in its valence shell, it can lose 7 electrons or gain 1 electron to attain stable configuration.
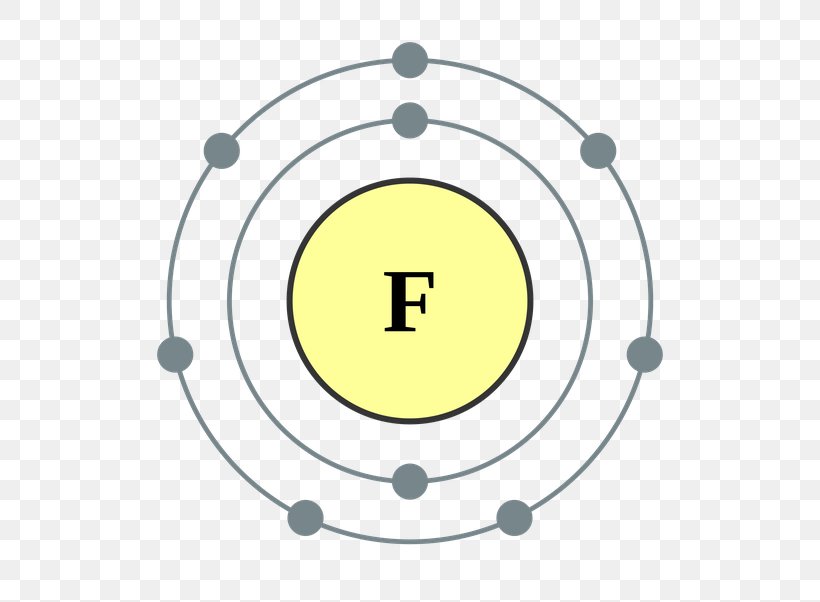
Atomic Fluorine Has 7 Valence Electrons;
It has 9 electrons, 2 core and 7 valence. There are six fluorine atoms that form bonds. Writing lewis structures and such things, fluorine always forms single bonds. Based on the electron configuration of fluorine, how many bonds does fluorine form even if it does not undergo hybridization?