How Many Bonds Can Helium Form
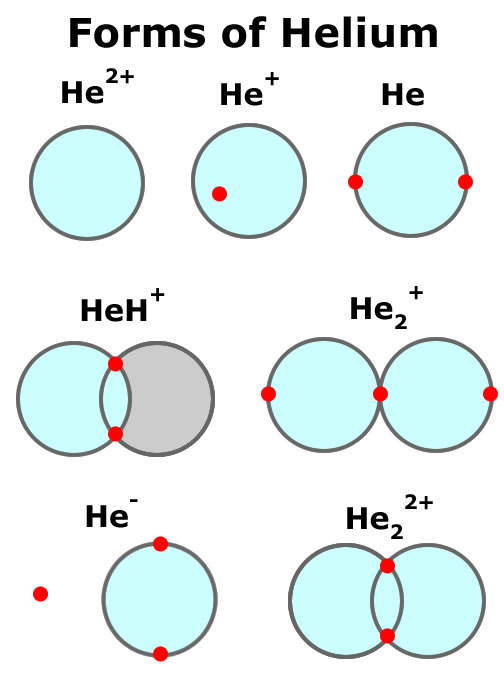
How Many Bonds Can Helium Form - These h − h molecules are themselves. Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen. How many hydrogen bonds can ch,o make to water? The energy required for breaking the bonds is as follows: It is a noble gas and is ths relatively. Web helium (he), neon (ne), and argon (ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. In order to bond it to another element, it would. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web after hydrogen, helium is the second most abundant element in the universe. Two hydrogen atoms can form covalent bond because they only have two electrons in.
How many hydrogen bonds can ch,o make to water? Web after hydrogen, helium is the second most abundant element in the universe. See answer (1) best answer. Web the two chlorine atoms are said to be joined by a covalent bond. These h − h molecules are themselves. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along. The energy required for breaking the bonds is as follows: It is present in all stars. There is only one shell of electrons, the valence shell of two electrons. This makes them highly stable as single atoms.
This makes them highly stable as single atoms. Web after hydrogen, helium is the second most abundant element in the universe. Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web the two chlorine atoms are said to be joined by a covalent bond. Web helium already has 2, so it will typically make zero bonds. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web each h can form only a single covalent bond, leading to the formation of h − h molecules, which are often also written as h 2 molecules. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along.
representation molecule (Lewis, valence)
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web a helium atom is an atom of the chemical element helium. It is a noble gas and is ths relatively. The energy required for breaking the bonds is as follows: In order to bond it to another element, it.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Two hydrogen atoms can form covalent bond because they only have two electrons in. Web after hydrogen, helium is the second most abundant element in the universe. Web helium already has 2, so it will typically make zero bonds. Web the two chlorine atoms are said to be joined by a covalent bond. Web how many covalent bonds does helium.
Vast new reserves of helium discovered Cosmos Chemistry education
It is a noble gas and is ths relatively. Web helium already has 2, so it will typically make zero bonds. How many bonds can helium form, helium: Web each h can form only a single covalent bond, leading to the formation of h − h molecules, which are often also written as h 2 molecules. Get the right answer,.
Helium Form Periodic Table of Elements Stock Vector Illustration of
Web after hydrogen, helium is the second most abundant element in the universe. Web helium already has 2, so it will typically make zero bonds. Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot? Web the two chlorine atoms are said to be joined by a covalent bond. There is a quick way to work.
How to Predict number of bonds each element forms ChemSimplified
See answer (1) best answer. The energy required for breaking the bonds is as follows: There is only one shell of electrons, the valence shell of two electrons. It is present in all stars. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
How Many Valence Electrons Does Helium (He) Have? [Valency of He]
Web how many covalent bonds does helium form? Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot? Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web of all the elements, helium is the least reactive, so it does not form compounds under ordinary.
Hydrogen energy levels are not quite exact.
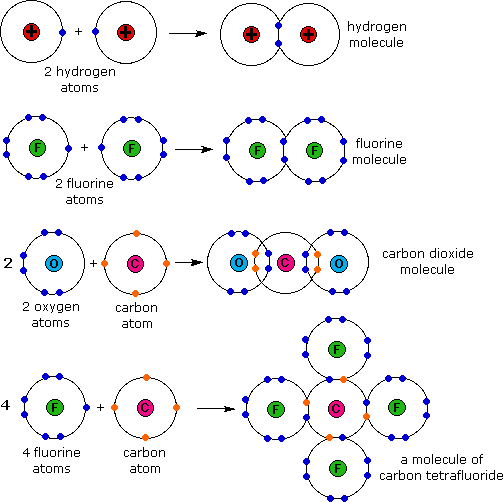
We can see this in h2 (hydrogen gas), h2o (water), and hcl (hydrochloric acid). Web helium already has 2, so it will typically make zero bonds. Upvote • 0 downvote add comment report still looking for help? Since ch2o has two hydrogen atoms hence it can form two hydrogen bond with oxygen. Web how many covalent bonds does helium form?
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web each h can form only a single covalent bond, leading to the formation of h − h molecules, which are often also written as h 2 molecules. This makes them highly stable as single atoms. Web of all the elements, helium is the least reactive, so it does not form compounds under ordinary conditions. Upvote • 0 downvote add.
Up, up and away Chemists say 'yes,' helium can form compounds
This makes them highly stable as single atoms. Web helium (he), neon (ne), and argon (ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. Web after hydrogen, helium is the second most abundant element in the universe. These h − h molecules are themselves. How many hydrogen bonds can ch,o make to.
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along. Web a helium atom is an atom of the chemical element helium. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Since ch2o has two hydrogen atoms hence it can form two hydrogen.
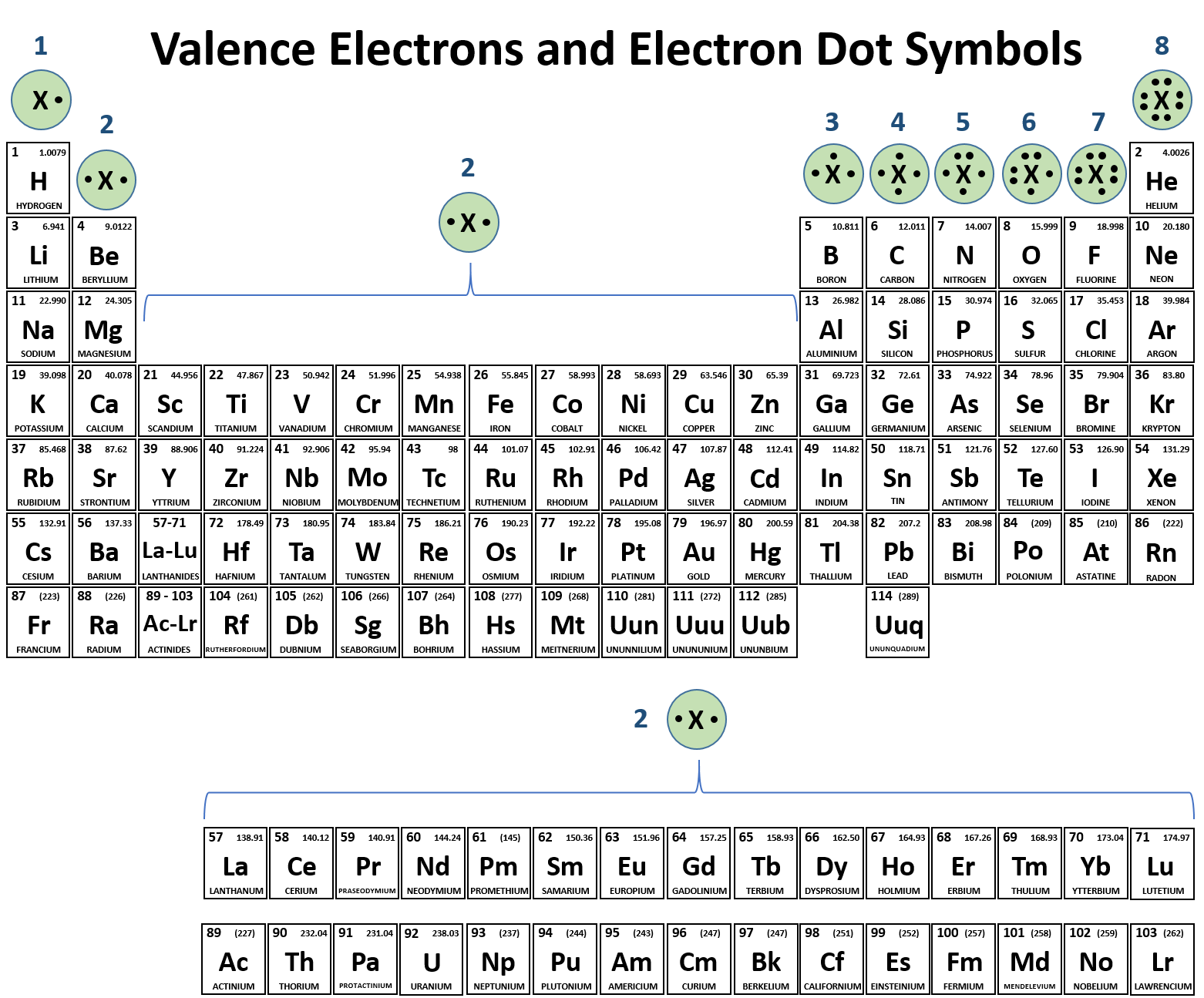
Web Helium (He), Neon (Ne), And Argon (Ar), As Group 18 Elements, Have Outer Electron Shells That Are Full Or Satisfy The Octet Rule.
Web metallic bonds why can hydrogen atoms form covalent bonds, but helium atoms cannot? How many hydrogen bonds can ch,o make to water? There is a quick way to work out how many covalent bonds an element will. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
In Each Case Hydrogen Will Share With The Other.
The energy required for breaking the bonds is as follows: Web a helium atom is an atom of the chemical element helium. In order to bond it to another element, it would. The reason that the two chlorine atoms stick together is that the shared pair of electrons is attracted.
Helium Atoms Have Two Electrons And Two Protons.
Web each h can form only a single covalent bond, leading to the formation of h − h molecules, which are often also written as h 2 molecules. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web hydrogen can only form one covalent bond. See answer (1) best answer.
Web The Two Chlorine Atoms Are Said To Be Joined By A Covalent Bond.
Get the right answer, fast. It is a noble gas and is ths relatively. There is only one shell of electrons, the valence shell of two electrons. Web how many covalent bonds does helium form?




![How Many Valence Electrons Does Helium (He) Have? [Valency of He]](https://1.bp.blogspot.com/-YNupZqYfI0Y/YACG_7--PTI/AAAAAAAADO0/um0r48XLVG8WVR-Xg5krtG2wU-eD7H6JgCLcBGAsYHQ/s832/Screenshot%2B%252857%2529.png)