How Do Molecules Form
How Do Molecules Form - Covalent bond, hydrogen bond, ionic bond, covalent bond. When the atoms are from different elements, the. Bonding occurs when atoms collide the. Web this video discuses in a nutshell why atoms form bonds and combine to form molecules. Atoms from different elements can combine. Atoms form chemical bonds with one another in order to become. The term “atom” refers to the tiniest unit of matter. Web 1 2 3 4 atoms and molecules all matter in the universe is made up of different particles. Everything is made up of molecules and the thermodynamics of bond energies make it. When two molecules react with each other inside a cell, their atoms are rearranged,.
Bonding occurs when atoms collide the. Web the attractive force that joins two or more atoms. Atoms from different elements can combine. Web coupled chemical reactions cells must obey the laws of chemistry and thermodynamics. Covalent bond, hydrogen bond, ionic bond, covalent bond. In chemistry, there are two main types of particle that we need to know about. Web there are a few ways atoms bond together to form molecules, and how two or more elements can become a compound. Web answer (1 of 2): Most molecules have positive and negative electric charge, and in different places. Web in this animated tutorial, you will learn about why do atoms combine to form molecules.
Covalent bond, hydrogen bond, ionic bond, covalent bond. Atoms form molecules or chemical bonds to lower their energy and get stability. Atoms form chemical bonds with one another in order to become. Web molecules form so that the atoms of each element in the molecule become stable. When two molecules react with each other inside a cell, their atoms are rearranged,. Most molecules have positive and negative electric charge, and in different places. Web in this animated tutorial, you will learn about why do atoms combine to form molecules. Web the attractive force that joins two or more atoms. Bonding occurs when atoms collide the. Molecules form when atoms join together.
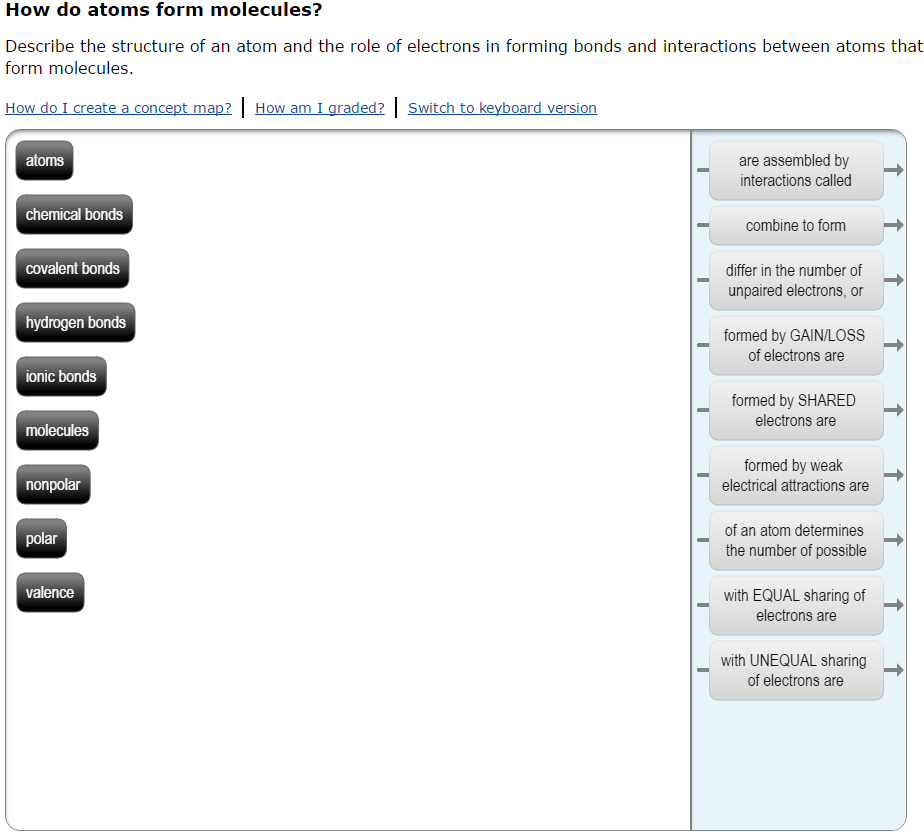
How do atoms form molecules? Describe the structure
Most molecules have positive and negative electric charge, and in different places. Atoms from different elements can combine. Three bonds that make atoms into molecules are. Covalent bond, hydrogen bond, ionic bond, covalent bond. Web a molecule is a collection of two or more atoms that are securely bound together by attractive forces or by chemical bonds.
Why do atoms form molecules? Quantum mechanical model of atoms. Arvin
Atoms form molecules or chemical bonds to lower their energy and get stability. Electrons are shared between two atoms. The term “atom” refers to the tiniest unit of matter. When two molecules react with each other inside a cell, their atoms are rearranged,. These bonds form as a result of the sharing or exchange of electrons among atoms.
A2U Molecule
Atoms form molecules or chemical bonds to lower their energy and get stability. In chemistry, there are two main types of particle that we need to know about. Web the attractive force that joins two or more atoms. Electrons are shared between two atoms. Atoms from different elements can combine.
11 Types of scientific changes with examples
Web molecules form so that the atoms of each element in the molecule become stable. Web molecules are made up of atoms that are held together by chemical bonds. Most molecules have positive and negative electric charge, and in different places. When two molecules react with each other inside a cell, their atoms are rearranged,. The molecules are held together.
How Do Atoms Come Together to Form Molecules? Sciencing
When two molecules react with each other inside a cell, their atoms are rearranged,. Web molecules are made when two or more atoms chemically bond together. Covalent bond, hydrogen bond, ionic bond, covalent bond. Molecules form when atoms join together. The molecules are held together by electrostatic forces.
Molecule Atom Element Atom, Chemical science, Molecules
The term “atom” refers to the tiniest unit of matter. Bonding occurs when atoms collide the. Web molecules are made up of atoms that are held together by chemical bonds. Web the attractive force that joins two or more atoms. Atoms form chemical bonds with one another in order to become.
Pengertian dan Perbedaan Jenis Partikel Materi Atom, Molekul, dan Ion
Web in this animated tutorial, you will learn about why do atoms combine to form molecules. Web a molecule is a collection of two or more atoms that are securely bound together by attractive forces or by chemical bonds. Covalent bond, hydrogen bond, ionic bond, covalent bond. Web answer (1 of 2): Molecules form when atoms join together.
How do molecules grow into crystals? UTokyo Research
Molecules form when atoms join together. The molecules are held together by electrostatic forces. Covalent bond, hydrogen bond, ionic bond, covalent bond. When two molecules react with each other inside a cell, their atoms are rearranged,. Atoms form molecules or chemical bonds to lower their energy and get stability.
Ionic Bond Vector Illustration Labeled Diagram With Formation
Web the attractive force that joins two or more atoms. The molecules are held together by electrostatic forces. Web a molecule is a collection of two or more atoms that are securely bound together by attractive forces or by chemical bonds. Everything is made up of molecules and the thermodynamics of bond energies make it. Covalent bond, hydrogen bond, ionic.
Molecule Easy Science Molecules, Science flashcards, Chemical changes
Web coupled chemical reactions cells must obey the laws of chemistry and thermodynamics. Molecules form when atoms join together. Atoms form chemical bonds with one another in order to become. Web molecules form so that the atoms of each element in the molecule become stable. Web 1 2 3 4 atoms and molecules all matter in the universe is made.
Web This Video Discuses In A Nutshell Why Atoms Form Bonds And Combine To Form Molecules.
Web answer (1 of 2): Web 1 2 3 4 atoms and molecules all matter in the universe is made up of different particles. Web the attractive force that joins two or more atoms. Web molecules are made when two or more atoms chemically bond together.
These Bonds Form As A Result Of The Sharing Or Exchange Of Electrons Among Atoms.
Web large biological molecules often assemble via dehydration synthesis reactions, in which one monomer forms a covalent bond to another monomer (or growing chain of. Three bonds that make atoms into molecules are. When two molecules react with each other inside a cell, their atoms are rearranged,. Atoms form molecules or chemical bonds to lower their energy and get stability.
Most Molecules Have Positive And Negative Electric Charge, And In Different Places.
Covalent bond, hydrogen bond, ionic bond, covalent bond. Molecules form when atoms join together. The term “atom” refers to the tiniest unit of matter. In chemistry, there are two main types of particle that we need to know about.
Atoms From Different Elements Can Combine.
Web in this animated tutorial, you will learn about why do atoms combine to form molecules. The molecules are held together by electrostatic forces. Web there are a few ways atoms bond together to form molecules, and how two or more elements can become a compound. When the atoms are from different elements, the.