Form 482 Fda
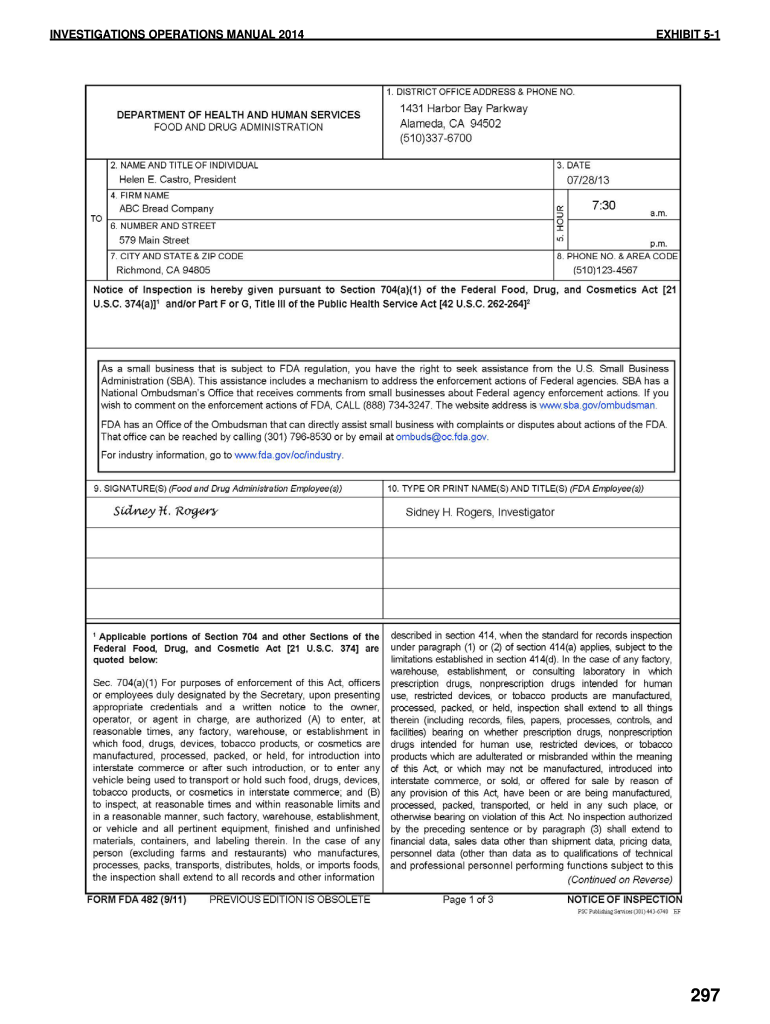
Form 482 Fda - Inspections covered by this compliance program involve facility inspections and data audits conducted under. Web what is an fda form 482? Sign it in a few clicks. Web what is the fda form 482? Use the following instructions to download the form if. Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Web do not issue the following forms: When the fda begins an inspection, a form 482 (notice of inspection) will be presented, along with contact information in the event a 483 response. Web usfda inspection form 482, form 483 & form 484 4.48 (23 ratings) usfda inspection form 482, form 483 & form 484 categories free engineering course, free. Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at.
Web what is the fda form 482? Web fda form 482 essentially provides notice of an fda audit to the manufacturing facility. Draw your signature, type it,. Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at. Investigator may tour the facility to get an idea of layout, workflow, and areas. Web do not issue the following forms: As per food and drug cosmetic act. Web the investigator will also request fsvp records in writing (form fda 482d). Learn about form 482 & 483. It is an official notice of fda for inspection signed by the fda officials.
Inspections covered by this compliance program involve facility inspections and data audits conducted under. Draw your signature, type it,. Web fda form 482 essentially provides notice of an fda audit to the manufacturing facility. It is produced by the inspector and has the authority to inspect the. Web do not issue the following forms: Web the investigator will also request fsvp records in writing (form fda 482d). Web what is an fda form 482? Learn about form 482 & 483. Web during the inspection. As per food and drug cosmetic act.
Form 48206510720114F Download Fillable PDF or Fill Online Air
Edit your fda form 482 online. Investigator may tour the facility to get an idea of layout, workflow, and areas. Web during the inspection. Web this is an example of form fda 482, notice of inspection what happens during the inspection? How serious is an fda 483?
Audit monitoring and inspections cro perspectives
Insanitary related content what should i expect during an inspection? Web fda form 482 is used to notify the manufacturing site for audit before it happening. Web • fda form 482c. It is produced by the inspector and has the authority to inspect the. An fda form 483 is issued to firm management at the conclusion of an inspection when.
what is FDA 482 and FDA 484 and other form used in FDA inspection Key
The exception is when conducting. How serious is an fda 483? Inspections covered by this compliance program involve facility inspections and data audits conducted under. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Edit your fda form 482 online.
Form FDA 3613a Supplementary Information Certificate of Exportability
Insanitary related content what should i expect during an inspection? As per food and drug cosmetic act. Learn about form 482 & 483. Use the following instructions to download the form if. Web do not issue the following forms:
Form 482 Fill out & sign online DocHub
Insanitary related content what should i expect during an inspection? Draw your signature, type it,. Edit your fda form 482 online. Web what is an fda form 482? Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of.
Form 482 Fill Out and Sign Printable PDF Template signNow
As per food and drug cosmetic act. Investigator may tour the facility to get an idea of layout, workflow, and areas. Sign it in a few clicks. | fda dec 21, 2020 — the investigator will. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that.
Form FDA 2830 Blood Establishment Registration and Product Listing
Use the following instructions to download the form if. Edit your fda form 482 online. How serious is an fda 483? Insanitary related content what should i expect during an inspection? Type text, add images, blackout confidential details, add comments, highlights and more.
Customs & International Trade Law Expert483 Inspection Observation
Web • fda form 482c. It is an official notice of fda for inspection signed by the fda officials. Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Insanitary related content what should i expect during an inspection? Draw your.
Form FDA 3542 Patent Information Submitted upon/after Approval of an
Investigator may tour the facility to get an idea of layout, workflow, and areas. Web what is an fda form 482? Web an fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a survey, or a response to a. Learn about form 482 & 483. Web during the inspection.
Type Text, Add Images, Blackout Confidential Details, Add Comments, Highlights And More.
Web usfda inspection form 482, form 483 & form 484 4.48 (23 ratings) usfda inspection form 482, form 483 & form 484 categories free engineering course, free. An fda form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that in their judgment may constitute. Web what is an fda form 482? Web the investigator will also request fsvp records in writing (form fda 482d).
Web • Fda Form 482C.
Use the following instructions to download the form if. As per food and drug cosmetic act. Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at. Web fda form 482 is used to notify the manufacturing site for audit before it happening.
Insanitary Related Content What Should I Expect During An Inspection?
It is produced by the inspector and has the authority to inspect the. Once the inspector arrives at a site, the host should review the inspector’s credentials and will receive a fda form 482, “notice of. | fda dec 21, 2020 — the investigator will. Draw your signature, type it,.
Fda Form 482 Is Called A Notice Of Inspection Form.
Inspections covered by this compliance program involve facility inspections and data audits conducted under. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Web what will you learn. Investigator may tour the facility to get an idea of layout, workflow, and areas.