Chemistry Mole Template
Chemistry Mole Template - See more ideas about chemistry, teaching chemistry, chemistry classroom. Web template for stuffed moles mole day projects principles of chemistry cut out two pieces like this for the body sides and one piece like this for the bottom cut out four for the paws. Web template for making your mole. The number of particles in a substance can be found using the avogadro constant. 1 master with 6 layout slides. Web the mole is the unit for the amount of substance. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. Experimental measurements have determined that. Ready to use template with text/picture. How many moles of n a h c o 3 are in 27.5 g n a h c o 3?
Web chemistry uses a unit called mole. How many moles of n a h c o 3 are in 27.5 g n a h c o 3? Web it provides a specific measure of the number of atoms or molecules in a bulk sample of matter. Web moles of n atoms (2 × 6.022 × 1023 of n atoms) to form a mole of n2o. How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Guidelines one mole per student. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. Experimental measurements have determined that. Web 1 mole of carbon = \strike{6\times10^{\strike{23}}\times\frac{12}{\strike{6\times10^{\strike{23}}}}} = 12g : Web template for making your mole.
Web template for making your mole. A mole is an entity that helps us to match the particles of a given substance along with its mass. See more ideas about chemistry, teaching chemistry, chemistry classroom. Web template for stuffed moles mole day projects principles of chemistry cut out two pieces like this for the body sides and one piece like this for the bottom cut out four for the paws. Web 1 mole of carbon = \strike{6\times10^{\strike{23}}\times\frac{12}{\strike{6\times10^{\strike{23}}}}} = 12g : Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Ready to use template with text/picture. In science, this is usually molecules or. Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (kalsi 3 o 8 ), a mineral used in the.
31 Mole Worksheet 1 Education Template
Guidelines one mole per student. Web this ‘molecular template for powerpoint and google slides’ features: Web this free powerpoint template is compatible with all latest microsoft powerpoint versions and can be also used as google slides themes. See more ideas about chemistry, teaching chemistry, chemistry classroom. Web it provides a specific measure of the number of atoms or molecules in.
Mole Day Projects and Ideas Chemistry Pinterest Crafts, We and
Web this free powerpoint template is compatible with all latest microsoft powerpoint versions and can be also used as google slides themes. Given the following equation, complete the questions. Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (kalsi 3 o 8 ), a mineral used in.
How to Make a MOLE! YouTube
How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Web a mole is a very important unit of measurement that chemists use. Given the following equation, complete the questions. A mole is an entity that helps us to match the particles of a given substance along with its mass. See more ideas about.
Mole Chemistry Project. MolebleHome! Chemistry projects, Mole day
Web moles of n atoms (2 × 6.022 × 1023 of n atoms) to form a mole of n2o. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a. Web template for making your mole. A mole is an entity that helps us to match the particles of a given substance along with its mass..
Mole Stuffed Animal Project Stuffed mole project pattern Mole day
Web this ‘molecular template for powerpoint and google slides’ features: Web chemistry uses a unit called mole. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. See more ideas about chemistry, teaching chemistry, chemistry classroom. In science, this is usually.
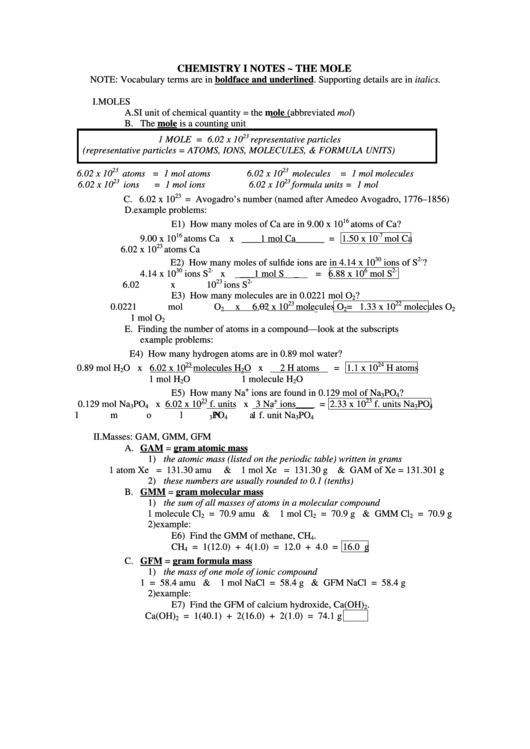
Chemistry Notes The Mole printable pdf download
The study of the numerical. Web this free powerpoint template is compatible with all latest microsoft powerpoint versions and can be also used as google slides themes. A mole is defined as the amount of substance containing the same number of. Web chemistry uses a unit called mole. How many grams of h 2 o 2 are in 2.0 mol.
29 best images about Chemistry mole project on Pinterest Student
How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Given the following equation, complete the questions. The number of particles in a substance can be found using the avogadro constant. Web explore professionally designed chemistry templates you can customize and share easily from canva. Web this ‘molecular template for powerpoint and google slides’.
Chemistry Mole Calculation Worksheet Interactive worksheet
Web explore professionally designed chemistry templates you can customize and share easily from canva. In science, this is usually molecules or. Experimental measurements have determined that. Web it provides a specific measure of the number of atoms or molecules in a bulk sample of matter. Web template for stuffed moles mole day projects principles of chemistry cut out two pieces.
What is a Chemistry Mole....Explained! YouTube
The mass of product depends upon the mass. Given the following equation, complete the questions. How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Web mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. Web explore.
Chemistry 1 Mole per Litre Chemistry Sticker TeePublic
How many grams of h 2 o 2 are in 2.0 mol h 2 o 2? Web a mole is a very important unit of measurement that chemists use. Web template for stuffed moles mole day projects principles of chemistry cut out two pieces like this for the body sides and one piece like this for the bottom cut out.
How Many Grams Of H 2 O 2 Are In 2.0 Mol H 2 O 2?
Web template for stuffed moles mole day projects principles of chemistry cut out two pieces like this for the body sides and one piece like this for the bottom cut out four for the paws. Web this free powerpoint template is compatible with all latest microsoft powerpoint versions and can be also used as google slides themes. A mole is defined as the amount of substance containing the same number of. How many moles of n a h c o 3 are in 27.5 g n a h c o 3?
In Science, This Is Usually Molecules Or.
1 master with 6 layout slides. A mole is an entity that helps us to match the particles of a given substance along with its mass. The study of the numerical. The mass of product depends upon the mass.
Guidelines One Mole Per Student.
Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. Experimental measurements have determined that. Web it provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a.
Submission Students Will Bring Their Mole To Class On Or Before The Due Date.
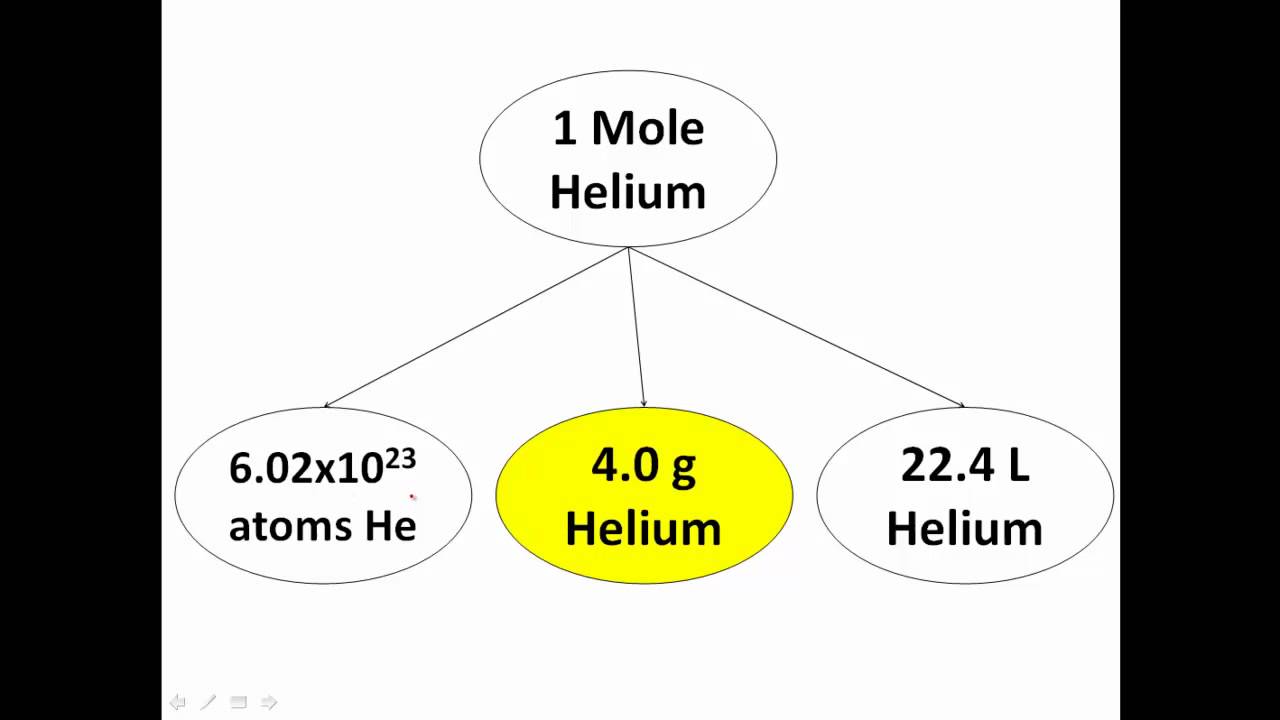
The number of particles in a substance can be found using the avogadro constant. Web updated on july 03, 2019 a mole is defined as a chemical unit, defined to be 6.022 x 10 23 ( avogadro's constant) entities. Web mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. Web this ‘molecular template for powerpoint and google slides’ features: