Chemistry Chapter 6
Chemistry Chapter 6 - F group 3a (3) cs i al click the card to flip 👆 1 electron gained 3 electrons lost 1. Why do some things form solutions and others not? Web bond length the average distance between the nuclei of two bonded atoms bond energy the energy required to break a chemical bond and form neutral isolated atoms electronegativity a measure of the ability of an atom in a chemical. Web we have compiled the ncert mcq questions for class 12 chemistry chapter 6 general principles and processes of isolation of elements with answers pdf free download covering the entire syllabus. Click the card to flip. Hydrogen bonding interactions and solubility 6.4: Gibbs energy and solubility 6.5: More about molecular composition pure substances mixtures unit 4: Web identify and define the three major types of chemical bonding. 1/2 of the distance between the nuclei of two atoms of the same element when atoms are joined.
Web terms in this set (55) chemical bonding. Web 1 / 32 flashcards learn test match created by emma_pear terms in this set (32) identify and define the three major types of chemical bonding in ionic bonding, large numbers of oppositely charged ions join because of. Web cbse class 11 chemistry chapter 6 notes helps to understands the basic laws governed in system and surroundings. Thermodynamics is a branch of science that deals with the relationship between heat and other forms of energy. Extraction of crude metal from concentrated ore 4. Web chemistry chapter 6 state the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Metallurgy is a scientific and technological process which is followed to. Ncert book for class 12 chemistry chapter 6. Gibbs energy and solubility 6.5: Class 11 thermodynamic notes contain the laws, different types of.
Web 11 class (1st year) chemistry chapter 6 notes. Energy required to move an electron from an atom. Chapter 6 chemistry\ 36 terms. Web chemistry chapter 6 homework. Web 1 / 50 flashcards learn match created by mallorybryant terms in this set (50) maximize stability and lower energy two reasons that elements want to bond noble gases elements want their electron configuration to look like these elements dash symbolizes a bond in the lewis structure two number of elements represented by a dash in the lewis. Ce4+ (aq) + fe2+ (aq) ce3+ (aq) + fe3+ (aq) click the card to flip 👆. Extraction of crude metal from concentrated ore 4. These notes are available in pdf and free of cost so that you can download chapter. Why do some things form solutions and others not? More about molecular composition pure substances mixtures unit 4:
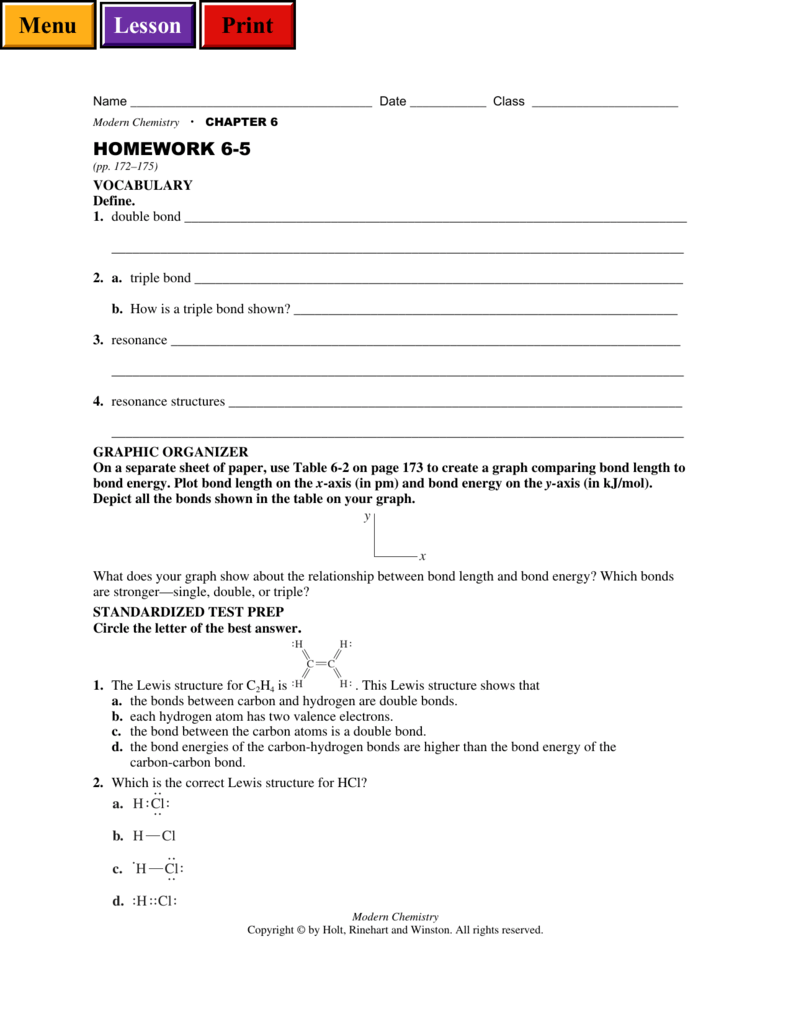
Modern Chemistry CHAPTER 6 HOMEWORK 65
Extraction of crude metal from concentrated ore 4. Web bond length the average distance between the nuclei of two bonded atoms bond energy the energy required to break a chemical bond and form neutral isolated atoms electronegativity a measure of the ability of an atom in a chemical. Ce4+ (aq) + fe2+ (aq) ce3+ (aq) + fe3+ (aq) click the.
PPT Modern Chemistry Chapter 6 Chemical Bonding PowerPoint
Chemical bonding that results from the. You will get the full notes of intermediate 11 class chemistry chapter # 6, chemical bonding. Chapter 6 chemistry\ 36 terms. Web identify and define the three major types of chemical bonding. Web chemistry chapter 6 homework.
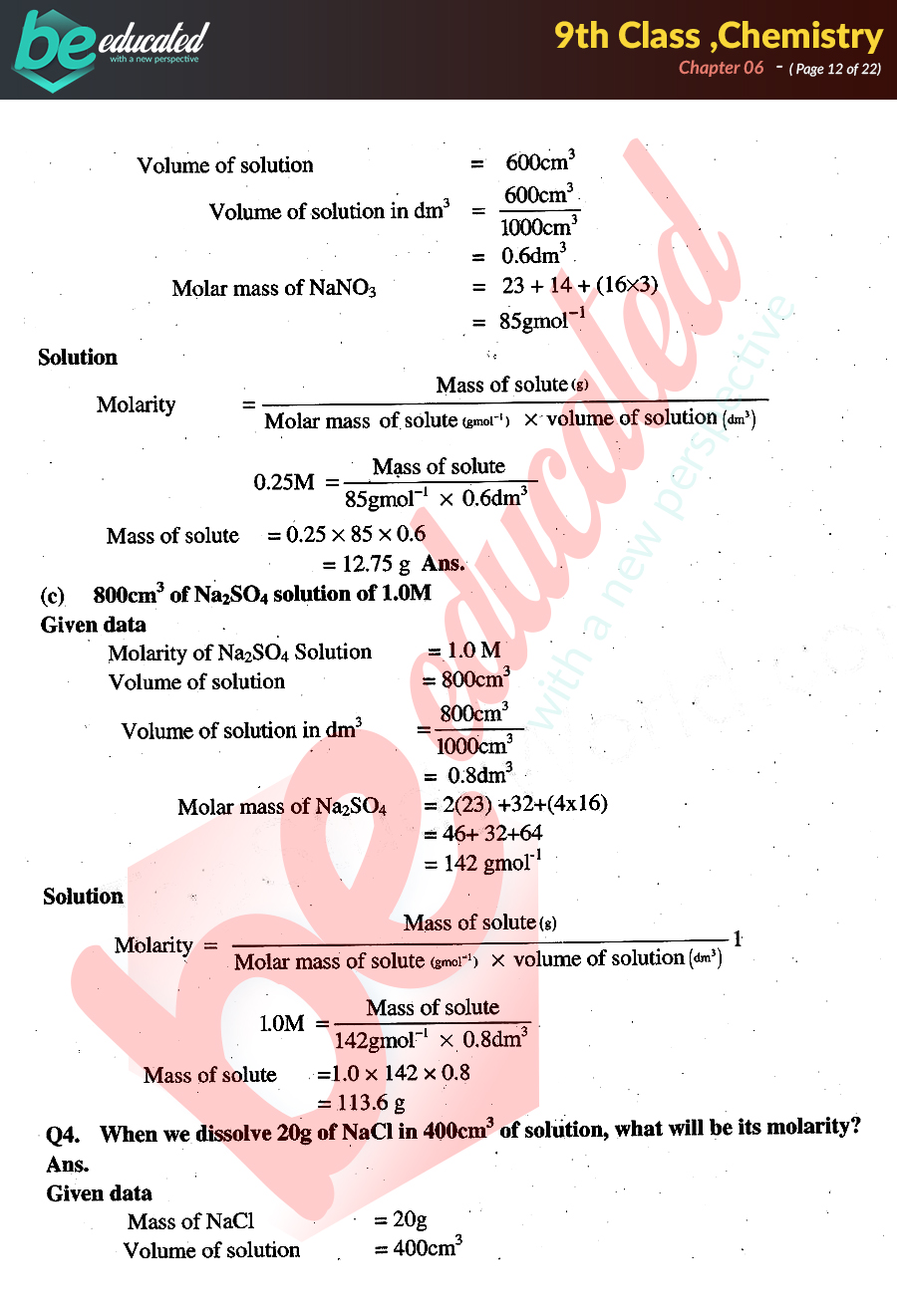
Chapter 6 Chemistry 9th Class Notes Matric Part 1 Notes
A substance that forms in a chemical reaction. Web chapter 6 chemistry flashcards | quizlet. Ncert solutions class 11 chemistry chemistry lab manual chemistry sample papers ncert class 11 chemistry. Thermodynamics is a branch of science that deals with the relationship between heat and other forms of energy. New products atoms are not created, and old reactant atoms are not.
NCERT Solutions for Class 11 Chemistry Chapter 6 Thermodynamics Free PDF
Ce4+ (aq) + fe2+ (aq) ce3+ (aq) + fe3+ (aq) click the card to flip 👆. Mutual electrical attraction between the nuclei and valence electrons or different atoms that binds the atoms together. Web topics and subtopics in ncert solutions for class 11 chemistry chapter 6 thermodynamics: Click the card to flip. Web 1 / 32 flashcards learn test match.
Integrated Physics and Chemistry Chapter 6 Activities Paradigm
These notes are available in pdf and free of cost so that you can download chapter. Web list out the topics/subtopics covered in chapter 6 of ncert solutions for class 12 chemistry. New products atoms are not created, and old reactant atoms are not destroyed. General principles and processes of isolation of elements. Why do some things form solutions and.
Chemistry Chapter 6 The Periodic Table Worksheet Answers
Web cbse class 11 chemistry chapter 6 notes helps to understands the basic laws governed in system and surroundings. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: Mutual electrical attraction between the nuclei and valence electrons or different atoms that binds the atoms together. General principles and processes of.
10th chemistry chapter 6 part 1 YouTube
Electrical attraction between cations and anions. More about atoms moles and molar mass isotopes unit 3: Web cbse class 11 chemistry chapter 6 notes helps to understands the basic laws governed in system and surroundings. These notes are available in pdf and free of cost so that you can download chapter. Web 11 class (1st year) chemistry chapter 6 notes.
35 Chemistry Chapter 6 The Periodic Table Worksheet Answers support
Hydrogen bonding interactions and solubility 6.4: Metallurgy is a scientific and technological process which is followed to. Ncert solutions class 11 chemistry chemistry lab manual chemistry sample papers ncert class 11 chemistry. Gibbs energy and solubility 6.5: Thermodynamics is a branch of science that deals with the relationship between heat and other forms of energy.
NCERT Exemplar Solutions Class 12 NCERT Exemplar Solutions General
F group 3a (3) cs i al click the card to flip 👆 1 electron gained 3 electrons lost 1. More about atoms moles and molar mass isotopes unit 3: More about molecular composition pure substances mixtures unit 4: General principles and processes of isolation of elements. Thermodynamics is a branch of science that deals with the relationship between heat.
Chemistry Chapter 6 Guided Reading Questions
Gibbs energy and solubility 6.5: Metallurgy is a scientific and technological process which is followed to. General principles and processes of isolation of elements. Click the card to flip. Web a substance or molecule that participates in a chemical reaction.
Chapter 6 Chemistry\ 36 Terms.
Ncert book for class 12 chemistry chapter 6. Web a substance or molecule that participates in a chemical reaction. Ce4+ (aq) + fe2+ (aq) ce3+ (aq) + fe3+ (aq) click the card to flip 👆. Electrical attraction between cations and anions.
Web List Out The Topics/Subtopics Covered In Chapter 6 Of Ncert Solutions For Class 12 Chemistry.
You will get the full notes of intermediate 11 class chemistry chapter # 6, chemical bonding. 1/2 of the distance between the nuclei of two atoms of the same element when atoms are joined. Practice mcq questions for class 12 chemistry. Why do some things form solutions and others not?
Mutual Electrical Attraction Between The Nuclei And Valence Electrons Or Different Atoms That Binds The Atoms Together.
Extraction of crude metal from concentrated ore 4. Web 1 / 32 flashcards learn test match created by emma_pear terms in this set (32) identify and define the three major types of chemical bonding in ionic bonding, large numbers of oppositely charged ions join because of. Ncert solutions class 11 chemistry chemistry lab manual chemistry sample papers ncert class 11 chemistry. Hydrogen bonding interactions and solubility 6.4:
Class 11 Thermodynamic Notes Contain The Laws, Different Types Of.
F group 3a (3) cs i al click the card to flip 👆 1 electron gained 3 electrons lost 1. Web chapter 6 practice test: Click the card to flip. Web we have compiled the ncert mcq questions for class 12 chemistry chapter 6 general principles and processes of isolation of elements with answers pdf free download covering the entire syllabus.