Chapter 7 Chemical Formulas And Chemical Compounds Answer Key
Chapter 7 Chemical Formulas And Chemical Compounds Answer Key - Polyatomic anions that contain oxygen. Atomic mass of each element. Chemical compounds and chemical formulas chapter interactives: Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. Web the number of atoms of each element contained in a single molecule of the compound. Covalent compounds naming and review practice with answer key… Web chemistry chapter 7 review (names and formulas) term. Atomic and mass number, avogadro number and mole, branches of chemistry, chemical calculations, elements and compounds particles, elements compounds and mixtures, empirical and molecular formulas, gram atomic mass molecular mass and gram formula… Write empirical formulas to match the following molecular. Common chemicals and their common names naming ionic and covalent compounds oxidation numbers oxidation numbers #2 valence electrons and oxidation state oxidation numbers molecular and empirical formulas
C) type of bond holding particles together in a compound. What is the purpose of an empirical formula? Bonds form between the atoms. Web chemistry chapter 7 review (names and formulas) term. What is the difference between a monatomic and a polyatomic ion? Web big idea chemical formulas represent the ratios of atoms in a chemical compound. Web atoms of that element in the compound. As 2 o 5 c. Web a) charge on an ion. Web write the chemical formulas for the following compounds:
Web atoms of that element in the compound. Determine the smallest angular velocity of the shaft if it is restricted not to twist more than 1°. Only the outer electrons move. Web the number of atoms of each element contained in a single molecule of the compound. Web chapter 7 chemical formulas and chemical compounds test answer key at the end of this section, you will be able to: A lattice of positive and negative ions held together by mutual attraction. D) distribution of electrons among the bonded particles in a compound. As 2 o 5 c. Web chemistry chapter 7 review (names and formulas) term. Web chapter 7 chapter 7 highlights 1.
Names and formulas of ionic compounds study guide
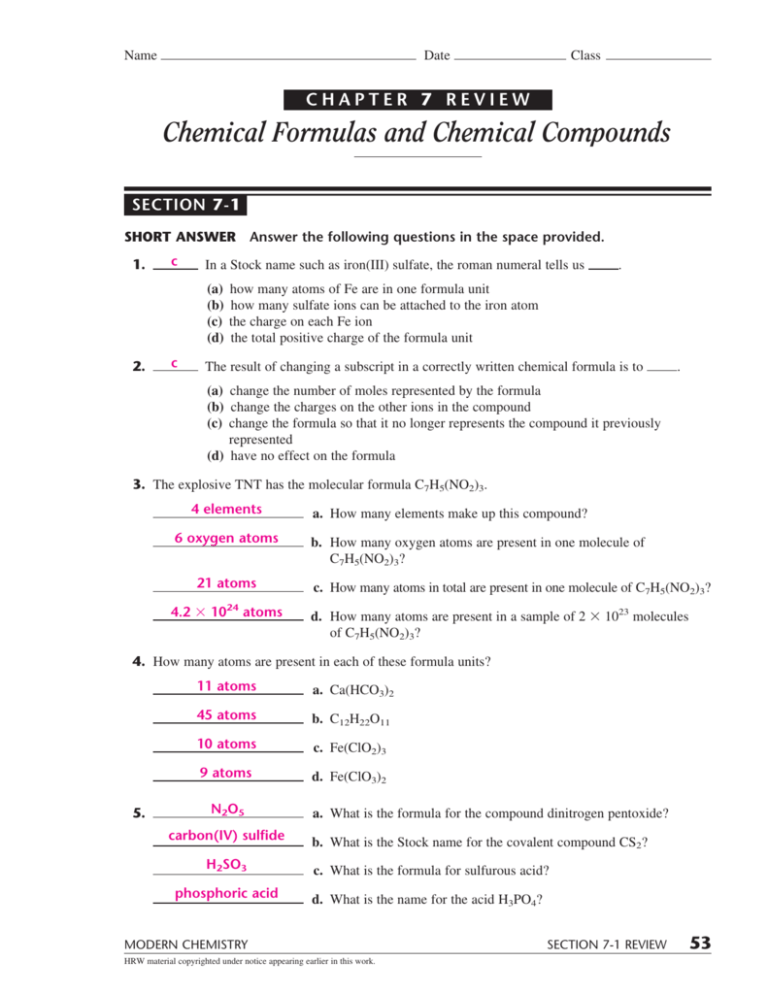
For each of the following ionic compounds… Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. Web chapter 7 review chemical formulas and chemical compounds section 4 short answer answer the following questions in the space provided. Only the outer electrons.
Chapter 7 Chemical Formulas And Chemical Compounds Answer Key
The simplest ratio of the compound's. Covalent compounds naming and review practice with answer key… For each of the following ionic compounds… Chemical nomenclature 7.1 molecular formula practice questions use the link below to answer the following questions:. Web chapter 7 chapter 7 highlights 1.
Worksheet Chemical Formula Writing Worksheet Worksheet Ionic —
Web adv chem resource nearpod codes chapter seven: Define binary and ternary ionic compounds. Various rules are used to name ionic and covalent compounds. What is the difference between a monatomic and a polyatomic ion? Web chemistry mcq pdf book with answers, test 3 to solve mcq questions:
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
A lattice of positive and negative ions held together by mutual attraction. Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. What is the purpose of an empirical formula? B) number of atoms or ions in a compound. Determine the smallest.
Chemical Formulas and Chemical Compounds
Hydrocarbon (molecule with only h and c ). A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. It is required to transmit 35 kw of power from the engine e to the generator g. Covalent compounds naming and review practice with answer key… Click the card to flip 👆.
PPT Modern Chemistry Chapter 7 Chemical Formulas & Chemical Compounds
Web the number of atoms of each element contained in a single molecule of the compound. Ions with greater number of oxygen atoms ends in. C) type of bond holding particles together in a compound. Web test created by blonde10123 terms in this set (28) copper (ii) carbonate cuco₃ sodium sulfite na₂so₃ ammonium phosphate (nh₄)₃po₄ tin (iv) sulfide sns₂ nitrous.
Writing formulas Ionic Compounds Chem Worksheet 8 3 Answer Key
Polyatomic anions that contain oxygen. Web adv chem resource nearpod codes chapter seven: Web chapter 7 chapter 7 highlights 1. Web chapter 7 chemical formulas and chemical compounds test answer key at the end of this section, you will be able to: P, i, cl, and o would form.
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Determine the smallest angular velocity of the shaft if it is restricted not to twist more than 1°. Web test created by blonde10123 terms in this set (28) copper (ii) carbonate cuco₃ sodium sulfite na₂so₃ ammonium phosphate (nh₄)₃po₄ tin (iv) sulfide sns₂ nitrous acid hno₂ mg (clo₄)₂ magnesium perchlorate fe (no₃)₂ iron (ii) nitrate fe (no₂)₃ cobalt (ii) oxide coo.
Chapter 7.3 Using Chemical Formulas
The simplest ratio of the compound's. Covalent compounds naming and review practice with answer key… Write empirical formulas to match the following molecular. Number of atoms or ions of each element that are combined in the compound. P, i, cl, and o would form.
Chapter 7 Section 3 Using Chemical Formulas
Various rules are used to name ionic and covalent compounds. Click the card to flip 👆. Common chemicals and their common names naming ionic and covalent compounds oxidation numbers oxidation numbers #2 valence electrons and oxidation state oxidation numbers molecular and empirical formulas Web chemistry chapter 7 review (names and formulas) term. Define binary and ternary ionic compounds.
Various Rules Are Used To Name Ionic And Covalent Compounds.
Common chemicals and their common names naming ionic and covalent compounds oxidation numbers oxidation numbers #2 valence electrons and oxidation state oxidation numbers molecular and empirical formulas Web chemistry chapter 7 review (names and formulas) term. Click the card to flip 👆. Web test created by blonde10123 terms in this set (28) copper (ii) carbonate cuco₃ sodium sulfite na₂so₃ ammonium phosphate (nh₄)₃po₄ tin (iv) sulfide sns₂ nitrous acid hno₂ mg (clo₄)₂ magnesium perchlorate fe (no₃)₂ iron (ii) nitrate fe (no₂)₃ cobalt (ii) oxide coo nitrogen (v) oxide how many atoms are represented by the formula.
What Is The Purpose Of An Empirical Formula?
Polyatomic anions that contain oxygen. Symbolize the composition of molecules that use molecular formulas and empirical formulas represent the arrangement of bonding of atoms within molecules that use structural formula a molecular formula. Web chapter 7 practice test with answer key.pdf. Only the outer electrons move.
A Lattice Of Positive And Negative Ions Held Together By Mutual Attraction.
Ions with greater number of oxygen atoms ends in. Covalent compounds naming and review practice with answer key… Number of atoms or ions of each element that are combined in the compound. Web chapter 7 chapter 7 highlights 1.
Positive Charges Form When Electrons Are Lost.
Ionic compounds are composed to elements in an _______________ bond. B 4 h 10 answers 1. C) type of bond holding particles together in a compound. Web chapter 7 chemical formulas and chemical compounds test answer key at the end of this section, you will be able to: