Chapter 6 The Periodic Table And Periodic Law
Chapter 6 The Periodic Table And Periodic Law - Include contributions made by lavoisier, newlands, mendeleev, and moseley. Web start studying chapter 6: Web 1/16 previous ← next → flip space created by ambssbabyy123 chemistry test review terms in this set (16) periodic law statement that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. When the elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties. The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. The periodic table and periodic law. The periodic table and periodic law in this chapter: Table 6.2 summarizes the contributions of. Web chapter 6 the periodic table and periodic law section 6.1 development of the pt 1. Click the card to flip 👆.
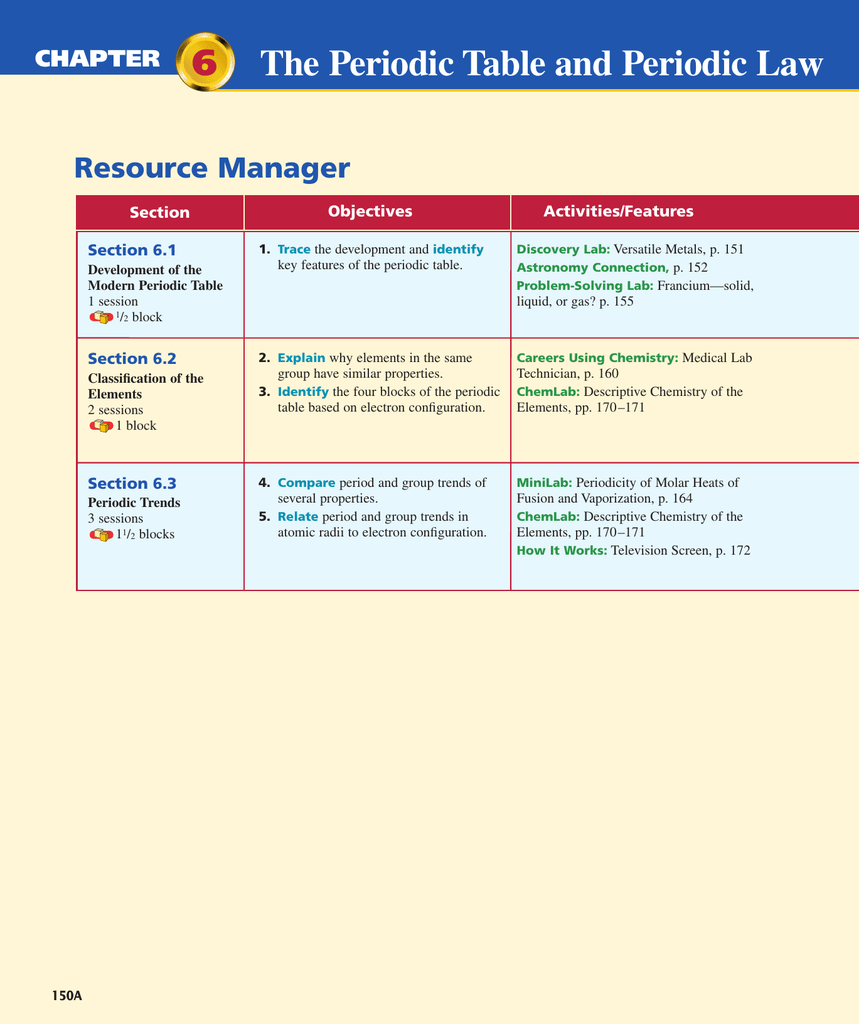
Table 6.2 summarizes the contributions of. The arrangement of the periodic table is based on the number of: Representative elements group a elements transition elements group b elements The periodic table and periodic law in this chapter: Explain why elements in a group have similar properties. When interpreted properly, the table describes much of the elements physical. Web increasing atomic number is called the periodic law. The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. Protons in the nucleus of an atom of the element. Web periodic law (6.1) statement that there is a periodic repetition of chemical and physical properties of the elements when arranged by increasing atomic number.
Protons in the nucleus of an atom of the element. Web problem 1 describe the development of the modern periodic table. He discovered that the properties of elements repeat in a periodic way. Representative elements group a elements transition elements group b elements Web 1/16 previous ← next → flip space created by ambssbabyy123 chemistry test review terms in this set (16) periodic law statement that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. The periodic table and periodic law. The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. When interpreted properly, the table describes much of the elements physical. Group 1 a elements, except for hydrogen, that are on the left side of the modern periodic. Relate the group and period trends seen in.
Chemistry Chapter 6 The Periodic Table Worksheet Answers
Include contributions made by lavoisier, newlands, mendeleev, and moseley. He discovered that the properties of elements repeat in a periodic way. Web problem 1 describe the development of the modern periodic table. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. Learn vocabulary, terms, and more with flashcards, games, and other study.
Chapter 6 The Periodic Table and Periodic Law
Web periodic law (6.1) statement that there is a periodic repetition of chemical and physical properties of the elements when arranged by increasing atomic number. He discovered that the properties of elements repeat in a periodic way. Web chapter 6 the periodic table and periodic law vocabulary. Identify different key features of the periodic table. Web chapter 6 the periodic.
Chapter 6 Study Guide The Periodic Table And Periodic Law Study Poster
Learn vocabulary, terms, and more with flashcards, games, and other study tools. Table 6.2 summarizes the contributions of. The periodic table and periodic law in this chapter: Web tjdubinski terms in this set (26) periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their properties. Web problem 1 describe the.
Chapter 6 The Periodic Table And Periodic Law Study Guide Study Poster
The periodic table and periodic law. Identify different key features of the periodic table. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. Web 1/16 previous ← next → flip space created by ambssbabyy123 chemistry test review terms in this set (16) periodic law statement that when the elements are arranged by.
Chapter 6 The Periodic Table and Periodic Law
He discovered that the properties of elements repeat in a periodic way. Representative elements group a elements transition elements group b elements Relate the group and period trends seen in. Web tjdubinski terms in this set (26) periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their properties. Web periodic.
Chapter 6 Periodic Table CeilidhMavi
Web problem 1 describe the development of the modern periodic table. Include contributions made by lavoisier, newlands, mendeleev, and moseley. Web chapter 6 the periodic table and periodic law vocabulary. That when elements are arranged according to increasing atomic number there is a periodic repetition of their chemical and physical properties. Web periodic law (6.1) statement that there is a.
Chapter 6 Periodic table
Web video answers for all textbook questions of chapter 6, the periodic table and periodic law, glencoe chemistry by numerade What did newlands discovered (1865)? Click the card to flip 👆. Explain why elements in a group have similar properties. The periodic table and periodic law.
Chemistry Chapter 6 The Periodic Table Worksheet Answers
Lilia pronin numerade educator 01:12 problem 2 sketch a simplified. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;. Web 1/16 previous ← next → flip space created by ambssbabyy123 chemistry test review terms in this set (16) periodic law statement that when the elements are arranged by increasing atomic number, there.
Chapter 6 the periodic table
The periodic table and periodic law. The periodic table and periodic law. When the elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties. Web chapter 6 the periodic table and periodic law vocabulary. A column on the periodic table.
Chapter 6 The Periodic Table and Periodic Law
The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. Identify different key features of the periodic table. He discovered that the properties of elements repeat in a periodic way. Representative elements group a elements transition elements group b elements The periodic table and periodic law.
Web The Number Of Protons In The Nucleus Of An Atom.
Representative elements group a elements transition elements group b elements Web chapter 6 the periodic table and periodic law vocabulary. Web chapter 6 the periodic table and periodic law section 6.1 development of the pt 1. When the elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties.
Web Video Answers For All Textbook Questions Of Chapter 6, The Periodic Table And Periodic Law, Glencoe Chemistry By Numerade
Explain why elements in a group have similar properties. The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. Web increasing atomic number is called the periodic law. Group a vertical column of elements in the periodic table arranged in order of increasing atomic number;.
Relate The Group And Period Trends Seen In.
Properties of groups and periods in the late 1800s, russian chemist dmitri mendeleev created the periodic table by organizing elements by their atomic weight in increasing. When interpreted properly, the table describes much of the elements physical. Web start studying chapter 6: The arrangement of the periodic table is based on the number of:
Learn Vocabulary, Terms, And More With Flashcards, Games, And Other Study Tools.
Click the card to flip 👆. Lilia pronin numerade educator 01:12 problem 2 sketch a simplified. What did newlands discovered (1865)? Web problem 1 describe the development of the modern periodic table.