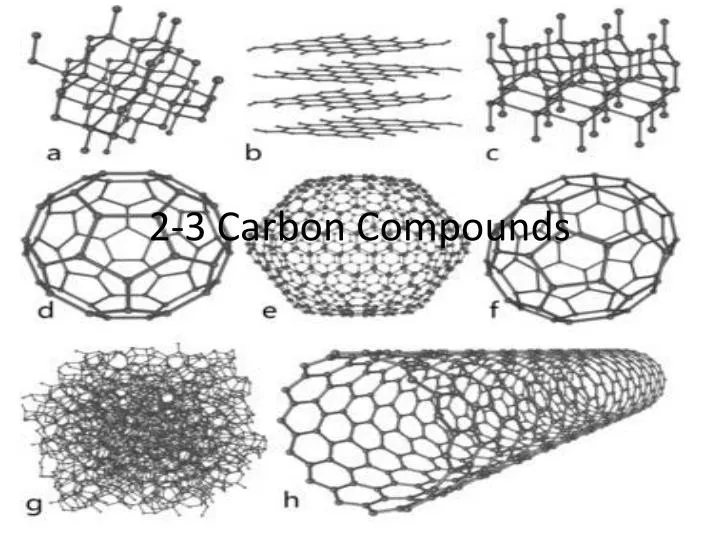
Chapter 2 Lesson 3 Carbon Compounds Answer Key
Chapter 2 Lesson 3 Carbon Compounds Answer Key - Web a compound made up of carbon , hydorgen and oxygen atoms;a type of nurient that is the major source of energy for the body. Web what properties of carbon explain its ability to form large and complex structures? Web objective 2.3.1describethe functions of each group of organic compounds. Carbon has 4 valence electrons allowing it to bond strongly with other elements. Web carbon forms a bond with what 4 elements? Look at the diagram of the general structure of an amino acid. Why does carbon have a whole branch of chemistry devoted to it? Biology 2 3 carbon compounds worksheet answers. Matter and energy 2.1 gq: Living things use carbohydrates as their main source of energy.
Why are the properties of water important to organisms? Web carbon forms a bond with what 4 elements? Oxygen, hydrogen, nitrogen, phosphorus, sulfer. How many oxygen atoms are found in the carboxyl group? Macromolecule made mostly from carbon and hydrogen atoms;includes fats, oils, and waxes. Color the same groups in the amino acids alanine and serine. Web chapter 2 big idea: What is the matter in organisms made of? Web carbon can bond with many elements including hydrogen (h), oxygen (o), phosphorous (p), sulphur (s), and nitrogen (n) to form the molecules of life. Carbon bonds with many elements, including hydrogen,.
Turn this in at the end of the period. Carbon bonds with many elements, including hydrogen,. Matter and energy 2.1 gq: Web macromolecule that contains carbon, hydrogen, oxygen, and nitrogen; Web compound made up of carbon, hydrogen, and oxygen atoms; Oxygen, hydrogen, nitrogen, phosphorus, sulfer. Why are the properties of water important to organisms? May be single, double, or triple bonds. Includes fats, oils and waxes. Describe the structures and functions of each of the four groups of macromolecules.
Important questions for class 10 Science Chapter 4 Carbon and its Compounds
Includes fats, oils and waxes. What does carbon bond with to make up life's molecules?. Giant molecule in living cell made of 1000's or up to 10000's of smaller molecules. It has 4 valence electrons to form strong covalent bonds. Web carbon can bond with many elements including hydrogen (h), oxygen (o), phosphorous (p), sulphur (s), and nitrogen (n) to.
Chapter 2 Lesson 3 Carbon Compounds Answer Key Exam Academy
Matter and energy 2.1 gq: Macromolecules containing hydrogen, oxygen, nitrogen, carbon. Chapter 2, the chemistry of life. Web macromolecule that contains carbon, hydrogen, oxygen, and nitrogen; Carbon bonds with many elements, including hydrogen,.
Chapter 2 Lesson 3 Carbon Compounds Answer Key MakenzieminMclean
Major source of energy for the human body. What is the matter in organisms made of? Includes fats, oils and waxes. How do organisms use different types of carbon compounds? Macromolecule made mostly from carbon and hydrogen atoms;includes fats, oils, and waxes.
PPT Section 23 Carbon Compounds PowerPoint Presentation, free
Look at the diagram of the general structure of an amino acid. The chart below shows key terms from the lesson. Major source of energy for the human body. Web carbon can bond with many elements including hydrogen (h), oxygen (o), phosphorous (p), sulphur (s), and nitrogen (n) to form the molecules of life. Color the carboxyl group blue.
Carbon Compound Form 5 4 Chemistry form 5 unit 2 CARBON COMPOUNDS
Web carbon can bond with many elements including hydrogen (h), oxygen (o), phosphorous (p), sulphur (s), and nitrogen (n) to form the molecules of life. Web chapter 2 lesson 3 “carbon compounds” directions: Carbon atoms can also bond to each other (this gives carbon. Web compound made up of carbon, hydrogen, and oxygen atoms; Web chapter 2 lesson 3 carbon.
Carbon Chemistry Worksheet Answers in 2020 Chemistry worksheets
Web chapter 2 lesson 3 “carbon compounds” directions: Turn this in at the end of the period. In the initial oval, they should write four groups of organic compounds. Describe the structures and functions of each of the four groups of macromolecules. Chapter 1, the science of biology;
NCERT Solutions for Class 10 Science Chapter 4 Carbon and its Compounds
Web chapter 2 big idea: Describe the unique qualities of carbon. What is the matter in organisms made of? Look at the diagram of the general structure of an amino acid. It can bond with itself multiple times and it has four valance electrons.
SECTION 2.1 THE CHEMISTRY OF LIFE Kha's Biology Portfolio
Section carbon compounds answer key. Carbon has 4 valence electrons allowing it to bond strongly with other elements. How many oxygen atoms are found in the carboxyl group? Web objective 2.3.1describethe functions of each group of organic compounds. Describe the structures and functions of each of the four groups of macromolecules.
23 Carbon Compounds Worksheet Answers Escolagersonalvesgui
How many oxygen atoms are found in the carboxyl group? Chapter 1, the science of biology; Turn this in at the end of the period. Why are the properties of water important to organisms? In the initial oval, they should write four groups of organic compounds.
PPT 23 Carbon Compounds PowerPoint Presentation, free download ID
It can bond with itself multiple times and it has four valance electrons. Macromolecules containing hydrogen, oxygen, nitrogen, carbon. Web a compound made up of carbon , hydorgen and oxygen atoms;a type of nurient that is the major source of energy for the body. Each electron can join with an electron from another atom to form a strong covalent bond..
Web Compound Made Up Of Carbon, Hydrogen, And Oxygen Atoms;
It can bond with itself multiple times and it has four valance electrons. Carbon atoms can also bond to each other (this gives carbon. Web a compound made up of carbon , hydorgen and oxygen atoms;a type of nurient that is the major source of energy for the body. Describe the unique qualities of carbon.
Why Are The Properties Of Water Important To Organisms?
Web what properties of carbon explain its ability to form large and complex structures? Matter and energy 2.1 gq: In the initial oval, they should write four groups of organic compounds. Why does carbon have a whole branch of chemistry devoted to it?
Web The Study Of Chemicals And Processes That Sustain Life.
Color the r group red. Web study with quizlet and memorize flashcards containing terms like carbon, collectively, most complex molecules based on carbon are known as _______., all other substances are ________(including a few small carbon. Describe the structures and functions of each of the four groups of macromolecules. The chart below shows key terms from the lesson.
Each Electron Can Join With An Electron From Another Atom To Form A Strong Covalent Bond.
Describe the structures and functions of each of the four groups of macromolecules. Vocabulary preview as students read, have them make a concept map using the section’s vocabulary terms, excluding the words monomer andpolymer. Carbon has 4 valence electrons allowing it to bond strongly with other elements. Major source of energy for the human body.