Barium Electron Configuration Long Form
Barium Electron Configuration Long Form - It is also known as abbreviated electron configuration. In this video we'll use the periodic. 1s 2 2s 2 2p 3: The 6th row, s block, 2nd column. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Members of a group typically have similar properties and electron configurations in their outer shell. Number of electrons (with no charge): Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and Let’s break down each method in detail.
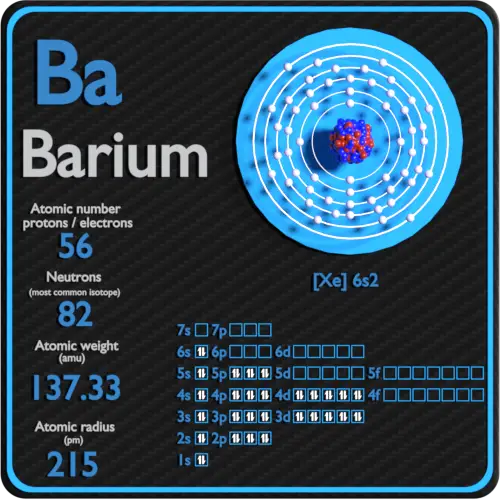
Web we can write the electron configuration of barium using four different methods: The first two groups (columns) of the periodic table represent the 's' orbital group. The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 6 6s 2. This means that the s,p,d,f electron configuration for barium must end with 6s^2. A horizontal row in the periodic table. Web the swedish chemist carl wilhelm scheele discovered (1774) a new base (baryta, or barium oxide, bao) as a minor constituent in pyrolusite, and from that base he prepared some crystals of barium sulfate, which he sent to johan gottlieb gahn, the discoverer of manganese. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): We’ll also look at why barium forms a 2+ ion and how the electron. 2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3:
Web how to write the electron configuration for barium. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: ← electronic configurations of elements. The 6th row, s block, 2nd column. Web we can write the electron configuration of barium using four different methods: 1s 2 2s 2 2p 3: The first two groups (columns) of the periodic table represent the 's' orbital group. It is also known as abbreviated electron configuration. Web electron configurations of the elements (data page) this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web there are 56 electrons for the barium atom.
Electron Configuration Of Barium cloudshareinfo
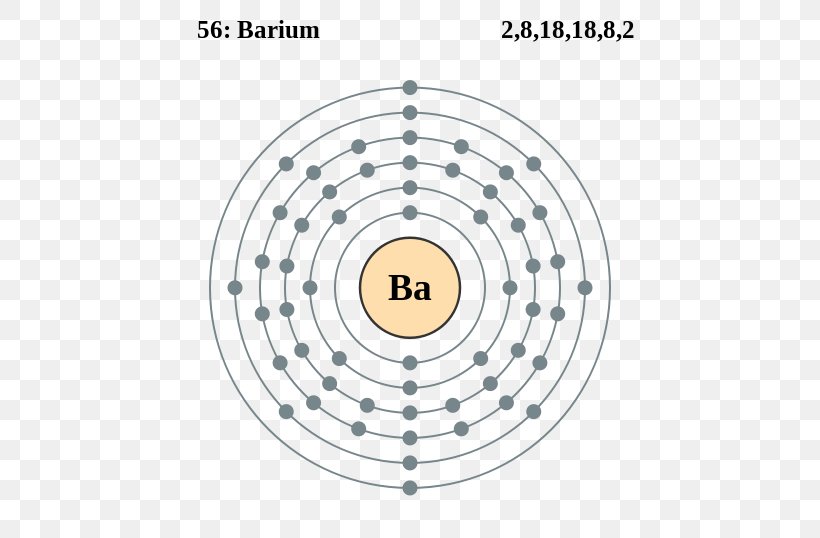
2, 8, 18, 18, 8, 2: Web the electron configuration of ba includes a total of 56 electrons. When we write the configuration, we'll put all 56 electrons in orbitals around the nucleus of the cobalt atom. Web we can write the electron configuration of barium using four different methods: The atomic number of each element increases by one, reading.
Barium Periodic Table and Atomic Properties
Number of electrons (with no charge): Web the atomic number of element barium is 56 and its electronic configuration can be written as: Possible oxidation states are +2. Electron configuration of oxygen (o. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and
Atomic structure of oxygen stock illustration. Illustration of physics
Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Members of a group typically have similar properties and electron configurations in their outer shell. Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and. Web electron.
Yeezy Quantum Barium Takes After a Chemical Element & 3 Other Yeezys!
Web electron configuration of barium. This means that the s,p,d,f electron configuration for barium must end with 6s^2. We’ll also look at why barium forms a 2+ ion and how the electron. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Possible oxidation states are +2.
Ba 2+ Electron Configuration (Barium Ion) YouTube
It is also in the 2nd group (column) of the periodic table. Electronic configuration of the barium atom in ascending order of orbital energies: The first two groups (columns) of the periodic table represent the 's' orbital group. Located in the vi period. Web a vertical column in the periodic table.
A stepbystep description of how to write the electron configuration
The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Number of electrons (with no charge): 25k views 2 years ago. Web an uncharged barium atom would have 56 electrons also. #1 using aufbau principle #2 using periodic table #3 from its bohr model #4 from its orbital diagram.
Barium electron configuration Newton Desk
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 cesium ← barium → lanthanum The unabbreviated electron configuration of ba is, 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Web electron configuration of barium is [xe] 6s2. Web we can write the electron configuration of barium using.
Symbol and electron diagram for barium Royalty Free Vector
2118 k (1845 °c, 3353 °f) density (near r.t.) 3.51 g/cm 3: When we write the configuration, we'll put all 56 electrons in orbitals around the nucleus of the cobalt atom. Web the electron configuration of ba includes a total of 56 electrons. 1000 k (727 °c, 1341 °f) boiling point: Web electron configuration of barium.
Electron Configuration Of Barium cloudshareinfo
#1 using aufbau principle #2 using periodic table #3 from its bohr model #4 from its orbital diagram. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Web electron configuration of barium is [xe] 6s2. Web how to write the electron configuration for barium..
56. Barium Quicycle Society
Web an uncharged barium atom would have 56 electrons also. Web the long form electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. This configuration shows that barium has six energy levels, and each level is filled with electrons. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 cesium.
For Each Atom The Subshells Are Given First In Concise Form, Then With All Subshells Written Out, Followed By The Number Of Electrons Per Shell.
25k views 2 years ago. The unabbreviated electron configuration of ba is, 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. 1s 2 2s 2 2p 1: The atomic number of each element increases by one, reading from left to right.
Web Electron Configuration 6S 2:
Web electron configuration of beryllium (be) [he] 2s 2: Located in the vi period. Electron configuration can be done in two ways. This means that the s,p,d,f electron configuration for barium must end with 6s^2.
The Electron Configuration Of Barium Is 1S2 2S2 2P6 3S2 3P6 3D10 4S2 4P6 4D10 5S2 5P6 6S2.
When liquid (at m.p.) 3.338 g/cm 3 : Possible oxidation states are +2. Web a vertical column in the periodic table. Electronic configuration of the barium atom in ascending order of orbital energies:
Web The Atomic Number Of Element Barium Is 56 And Its Electronic Configuration Can Be Written As:
1s 2 2s 2 2p 2: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 cesium ← barium → lanthanum 1000 k (727 °c, 1341 °f) boiling point: However as a group 2 element barium easily loses 2 electrons to form the +2 cation.