2010 Ap Chem Frq Form B
2010 Ap Chem Frq Form B - Essentially correct (e) if the conditional probability is correctly computed and. A galvanic cell and the balanced. Connecting students to college success. Identify the charge of the. This is meant as preparation for the ap exam but can also be done throughout. Web in fraction form is sufficient to be scored as essentially correct (e). Web ap®chemistry 2010 scoring guidelines (form b) question 2 (10 points) 5 fe2+(aq) + mno 4 (aq) + 8 h+(aq) o 5 fe3+(aq) + mn2+(aq) + 4 h 2o(l) a galvanic cell. (c) how many moles of. (i) calculate the molar concentration of oh−(aq) in the saturated solution. Part (b) is scored as follows:
Web in fraction form is sufficient to be scored as essentially correct (e). Identify the charge of the. Emeritus professor of mathematics metropolitan state university of denver june 5, 2017 1 problem 1 1.1. (b) calculate the value of the standard potential, e°, for the spontaneous cell reaction. A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using. Web ap® chemistry 2010 scoring guidelines (form b) © 2010 the college board. Web just ask if you need an explanation/solution to one of these. Part (b) is scored as follows: Web ap®chemistry 2010 scoring guidelines (form b) question 2 (10 points) 5 fe2+(aq) + mno 4 (aq) + 8 h+(aq) o 5 fe3+(aq) + mn2+(aq) + 4 h 2o(l) a galvanic cell. Connecting students to college success.
Essentially correct (e) if the conditional probability is correctly computed and. Question 6 (8 points) h 2 (g) + cl 2 (g) → 2 hcl(g) the table below gives data for a. Web ap®chemistry 2010 scoring guidelines (form b) question 2 (10 points) 5 fe2+(aq) + mno 4 (aq) + 8 h+(aq) o 5 fe3+(aq) + mn2+(aq) + 4 h 2o(l) a galvanic cell. One of my parents is an ap chem teacher, so these answers should be fairly accurate. In this series, we go over a decade's worth of ap chemistry frqs. Answer the following questions about the solubility and reactions of the ionic compounds m(oh)2 and mco3, where m represents an unidentified metal. Web (a) on the diagram, clearly label the cathode. Benzoic acid, c 6 h 5 cooh, dissociates in water as shown in the equation above. Web 2+ 1 point is earned for the correct charge. Web just ask if you need an explanation/solution to one of these.
AP Chemistry 2010 FRQ 5 YouTube
(c) how many moles of. Identify the charge of the. (i) calculate the molar concentration of oh−(aq) in the saturated solution. Web ap calculus 2010 ab (form b) frq solutions louis a. In this series, we go over a decade's worth of ap chemistry frqs.
2010 AP Chemistry FRQ number 2 YouTube
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. One of my parents is an ap chem teacher, so these answers should be fairly accurate. Web 2+ 1 point is earned for the correct charge. Web just ask if you need an explanation/solution to.
AP Calculus BC 2010 Form B FRQ 1 YouTube
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Question 6 (8 points) h 2 (g) + cl 2 (g) → 2 hcl(g) the table below gives data for a. Web in fraction form is sufficient to be scored as essentially correct (e). (b).
2002 AP Physics B FreeResponse Questions Form B
Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web just ask if you need an explanation/solution to one of these. Identify the charge of the. Web 2+ 1 point is earned for the correct charge. A galvanic cell and the balanced.
AP Calculus AB 2011 form B frq 5 part1 YouTube
Answer the following questions about the solubility and reactions of the ionic compounds m(oh)2 and mco3, where m represents an unidentified metal. (i) calculate the molar concentration of oh−(aq) in the saturated solution. Identify the charge of the. Benzoic acid, c 6 h 5 cooh, dissociates in water as shown in the equation above. A galvanic cell and the balanced.
2010 ap chem frq titrations YouTube
Web just ask if you need an explanation/solution to one of these. Answer the following questions about the solubility and reactions of the ionic compounds m(oh)2 and mco3, where m represents an unidentified metal. A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using. Web 2+ 1 point is earned for the correct charge. Web.
2010 (Form B) ABBC Calculus FRQ 4 YouTube
(b) calculate the value of the standard potential, e°, for the spontaneous cell reaction. Answer the following questions about the solubility and reactions of the ionic compounds m(oh)2 and mco3, where m represents an unidentified metal. A galvanic cell and the balanced. A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using. Web (a) on.
Calc AB 2010 (Form B) FRQ 2 YouTube
A galvanic cell and the balanced. Web (a) on the diagram, clearly label the cathode. Essentially correct (e) if the conditional probability is correctly computed and. This is meant as preparation for the ap exam but can also be done throughout. Web in fraction form is sufficient to be scored as essentially correct (e).
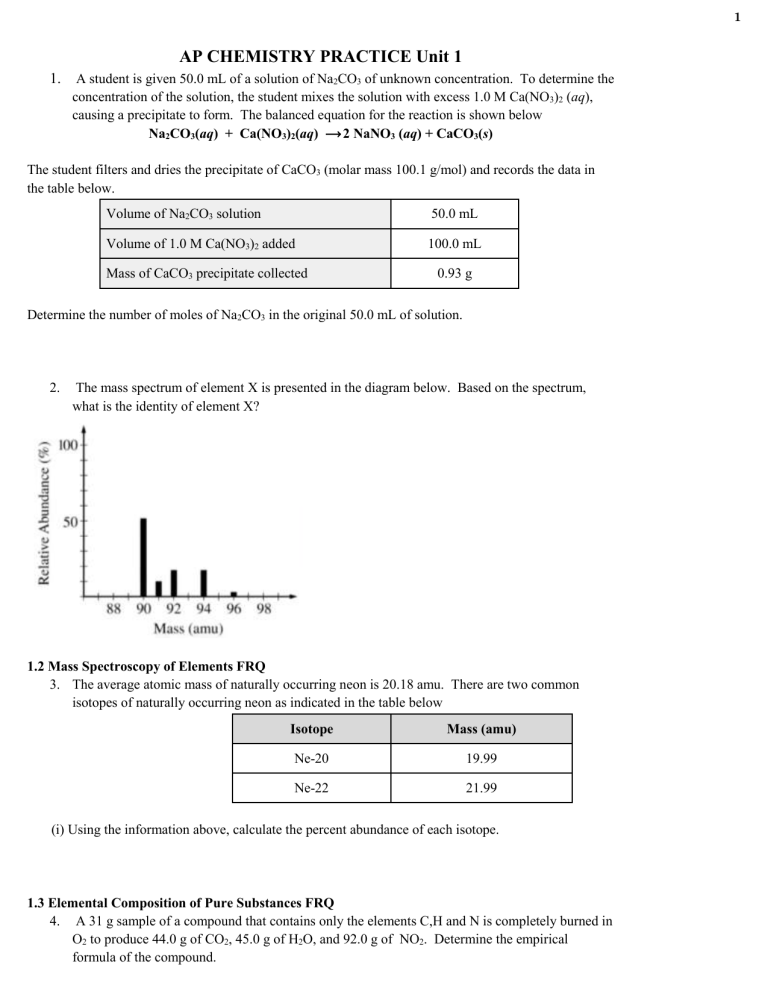
AP Chem Unit 1 FRQ practice
Part (b) is scored as follows: A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using. In this series, we go over a decade's worth of ap chemistry frqs. A galvanic cell and the balanced. Web ap calculus 2010 ab (form b) frq solutions louis a.
2010 AP Physics Free Response Answers Tests Standardized Tests
In this series, we go over a decade's worth of ap chemistry frqs. Identify the charge of the. At 25°c, a saturated solution of m(oh)2 has a ph of 9.15. A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using. Web ap calculus 2010 ab (form b) frq solutions louis a.
Emeritus Professor Of Mathematics Metropolitan State University Of Denver June 5, 2017 1 Problem 1 1.1.
(b) calculate the value of the standard potential, e°, for the spontaneous cell reaction. Web ap calculus 2010 ab (form b) frq solutions louis a. Web in fraction form is sufficient to be scored as essentially correct (e). At 25°c, a saturated solution of m(oh)2 has a ph of 9.15.
This Is Meant As Preparation For The Ap Exam But Can Also Be Done Throughout.
Web ap®chemistry 2010 scoring guidelines (form b) question 2 (10 points) 5 fe2+(aq) + mno 4 (aq) + 8 h+(aq) o 5 fe3+(aq) + mn2+(aq) + 4 h 2o(l) a galvanic cell. Web (a) on the diagram, clearly label the cathode. In this series, we go over a decade's worth of ap chemistry frqs. A 25.0 ml sample of an aqueous solution of pure benzoic acid is titrated using.
Question 6 (8 Points) H 2 (G) + Cl 2 (G) → 2 Hcl(G) The Table Below Gives Data For A.
One of my parents is an ap chem teacher, so these answers should be fairly accurate. Identify the charge of the. Web 2+ 1 point is earned for the correct charge. Essentially correct (e) if the conditional probability is correctly computed and.
Benzoic Acid, C 6 H 5 Cooh, Dissociates In Water As Shown In The Equation Above.
(i) calculate the molar concentration of oh−(aq) in the saturated solution. Part (b) is scored as follows: Answer the following questions about the solubility and reactions of the ionic compounds m(oh)2 and mco3, where m represents an unidentified metal. Web just ask if you need an explanation/solution to one of these.