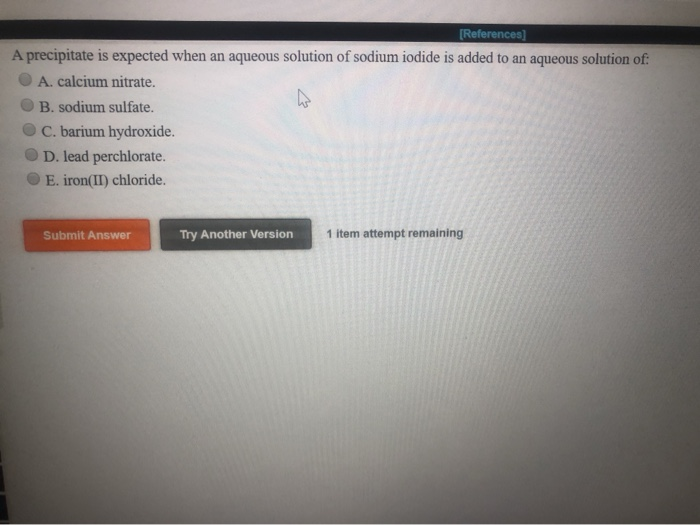
Which Anion Will Form A Precipitate With Al3+
Which Anion Will Form A Precipitate With Al3+ - Whether or not such a. With fe2+ fe 2 +, a dark blue precipitate is formed. Let’s look at an example of a precipitation reaction. Web which pair of ions would not be expected to form a precipitate when solutions are mixed? There are two possible reactions. Aluminium, al 3+ white precipitate: Web chemical separation by precipitation. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Web chemistry questions and answers. Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide.
Web potassium ferricyanide will give a brown coloration but no precipitate with fe3+ fe 3 +. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Click the card to flip 👆. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Which pair of ions would not be expected to form a precipitate when dilute solutions of each are mixed? Web chemistry questions and answers. Web 7 rows white precipitate remains: With fe2+ fe 2 +, a dark blue precipitate is formed. Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react.
Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. There are two possible reactions. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Web chemical separation by precipitation. Aluminium, al 3+ white precipitate: Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). Web 7 rows white precipitate remains:
Anion_Formation
Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Web potassium ferricyanide will give a brown coloration but no precipitate with fe3+ fe 3 +. Let’s look at an example of a precipitation reaction. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: A mixture.
Chimie, fizică și ecologie Identificarea cationilor (reacții de
With fe2+ fe 2 +, a dark blue precipitate is formed. There are two possible reactions. Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Aluminium, al 3+ white precipitate: Click the card to flip 👆.
How Does A Precipitate Form freelancegraphicdesigndetroit
Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Web 7 rows white precipitate remains: Let’s look at an example of a precipitation reaction. A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2.
PPT IONS PowerPoint Presentation ID2435906
Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. Let’s look at an example of a precipitation reaction. Web 7 rows white precipitate remains: Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Aluminium, al 3+ white precipitate:
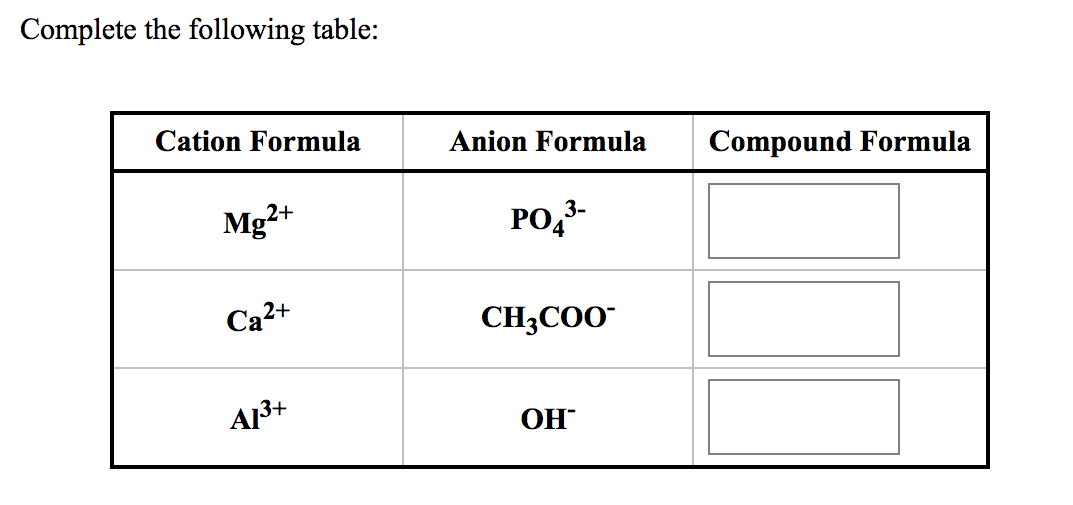
Solved Complete the following table Cation Formula Anion
A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,. Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Let’s look at an example of a precipitation.
Solved Experiment 11 PreLab Questions Nameca rmn Section
Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Let’s look at an example of a precipitation reaction. Click the card to flip 👆. Al 3+ (aq) + 3nh 3 (aq)+ 3h 2 o(aq) <==> al(oh) 3 (s) + 3nh 4 + (aq). Which.
Solved 3. For each of the following, identify the anion or
Web which pair of ions would not be expected to form a precipitate when solutions are mixed? Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. A type of quantitative chemical analysis that. Web precipitation reactions occur when cations and anions in aqueous solution.
Solved QUALITATIVE ANALYSIS OF ANIONS EXPERIMENT 13
Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Web 7 rows white precipitate remains: Aluminium, al 3+ white precipitate: Web chemical separation by precipitation.
Anion analysis POSTLABORATORY ASSIGNMENT 1. An iodide/chloride/sulfate
Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. Web chemical separation by precipitation. Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium.
Solved Which anion will form a precipitate with Ca2+? OH ОСІ
Whether or not such a. Web chemical separation by precipitation. Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Which pair of ions would not be expected to form a precipitate when dilute solutions of each are mixed? Al 3+ (aq) + 3nh 3 (aq)+ 3h.
There Are Two Possible Reactions.
Whether or not such a. Web aluminum ion reacts with aqueous ammonia to produce a white gelatinous precipitate of al(oh) 3: Click the card to flip 👆. A type of quantitative chemical analysis that.
Web Which Pair Of Ions Would Not Be Expected To Form A Precipitate When Solutions Are Mixed?
Web chemical separation by precipitation. Web precipitation is when a chemical reaction occurs between two solutions, and the reaction produces a product that is a solid. Web 7 rows white precipitate remains: A mixture of metal ions in a solution can be separated by precipitation with anions such as cl −, br −, so 4 2 −, co 3 2 −,.
Let’s Look At An Example Of A Precipitation Reaction.
Web a few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. Web zn2+ ions will react to produce a white precipitate of zinc hydroxide, and we get a similar reaction with al3+ ions, producing aluminum hydroxide. Web chemistry chemistry questions and answers question 5 (10 points) which anion will form a precipitate with a13+? Aluminium, al 3+ white precipitate:
Al 3+ (Aq) + 3Nh 3 (Aq)+ 3H 2 O(Aq) <==> Al(Oh) 3 (S) + 3Nh 4 + (Aq).
Web potassium ferricyanide will give a brown coloration but no precipitate with fe3+ fe 3 +. Reaction of ammonia with the hydroxonium ions (hydrogen ions) ammonia will react. Web chemistry questions and answers. Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.